Entrainment of neural oscillations as a modifiable substrate of attention
- PMID: 24630166
- PMCID: PMC4037370
- DOI: 10.1016/j.tics.2014.02.005
Entrainment of neural oscillations as a modifiable substrate of attention
Abstract
Brain operation is profoundly rhythmic. Oscillations of neural excitability shape sensory, motor, and cognitive processes. Intrinsic oscillations also entrain to external rhythms, allowing the brain to optimize the processing of predictable events such as speech. Moreover, selective attention to a particular rhythm in a complex environment entails entrainment of neural oscillations to its temporal structure. Entrainment appears to form one of the core mechanisms of selective attention, which is likely to be relevant to certain psychiatric disorders. Deficient entrainment has been found in schizophrenia and dyslexia and mounting evidence also suggests that it may be abnormal in attention-deficit/hyperactivity disorder (ADHD). Accordingly, we suggest that studying entrainment in selective-attention paradigms is likely to reveal mechanisms underlying deficits across multiple disorders.
Keywords: EEG; attention-deficit/hyperactivity disorder; dyslexia; entrainment; schizophrenia; selective attention.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures


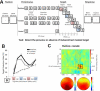
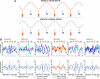
Similar articles
-
Low-frequency neuronal oscillations as instruments of sensory selection.Trends Neurosci. 2009 Jan;32(1):9-18. doi: 10.1016/j.tins.2008.09.012. Epub 2008 Nov 13. Trends Neurosci. 2009. PMID: 19012975 Free PMC article.
-
The role of abnormal neural oscillations in the pathophysiology of co-occurring Tourette syndrome and attention-deficit/hyperactivity disorder.Eur Child Adolesc Psychiatry. 2007 Jun;16 Suppl 1:51-9. doi: 10.1007/s00787-007-1007-3. Eur Child Adolesc Psychiatry. 2007. PMID: 17665283 Review.
-
Oscillatory recruitment of bilateral visual cortex during spatial attention to competing rhythmic inputs.J Neurosci. 2015 Apr 8;35(14):5489-503. doi: 10.1523/JNEUROSCI.2891-14.2015. J Neurosci. 2015. PMID: 25855167 Free PMC article.
-
"Can waiting awaken the resting brain?" A comparison of waiting- and cognitive task-induced attenuation of very low frequency neural oscillations.Brain Res. 2013 Aug 2;1524:34-43. doi: 10.1016/j.brainres.2013.05.043. Epub 2013 May 31. Brain Res. 2013. PMID: 23732340
-
Entrainment and synchronization of brain oscillations to auditory stimulations.Neurosci Res. 2020 Jul;156:271-278. doi: 10.1016/j.neures.2020.03.004. Epub 2020 Mar 19. Neurosci Res. 2020. PMID: 32201357 Review.
Cited by
-
Clarifying frequency-dependent brightness enhancement: delta- and theta-band flicker, not alpha-band flicker, consistently seen as brightest.Exp Brain Res. 2019 Aug;237(8):2061-2073. doi: 10.1007/s00221-019-05568-1. Epub 2019 Jun 6. Exp Brain Res. 2019. PMID: 31172241
-
Alignments between cortical neurochemical systems, proteinopathy and neurophysiological alterations along the Alzheimer's disease continuum.medRxiv [Preprint]. 2024 Apr 14:2024.04.13.24305551. doi: 10.1101/2024.04.13.24305551. medRxiv. 2024. PMID: 38645027 Free PMC article. Preprint.
-
A Temporal Sampling Basis for Visual Processing in Developmental Dyslexia.Front Hum Neurosci. 2020 Jul 8;14:213. doi: 10.3389/fnhum.2020.00213. eCollection 2020. Front Hum Neurosci. 2020. PMID: 32733217 Free PMC article.
-
Neural Entrainment vs. Stimulus-Tracking: A Conceptual Challenge for Rhythmic Perceptual Stimulation in Developmental Neuroscience.Front Psychol. 2022 May 6;13:878984. doi: 10.3389/fpsyg.2022.878984. eCollection 2022. Front Psychol. 2022. PMID: 35602682 Free PMC article. No abstract available.
-
Absence of Rhythm Benefit on Speech in Noise Recognition in Children Diagnosed With Auditory Processing Disorder.Front Neurosci. 2020 May 5;14:418. doi: 10.3389/fnins.2020.00418. eCollection 2020. Front Neurosci. 2020. PMID: 32477048 Free PMC article.
References
-
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. - PubMed
-
- Sporns O. Networks of the brain. MIT Press; 2011.
-
- Stone JL, Hughes JR. Early history of electroencephalography and establishment of the American Clinical Neurophysiology Society. J Clin Neurophysiol. 2013;30:28–44. - PubMed
-
- Thut G, et al. The functional importance of rhythmic activity in the brain. Curr Biol. 2012;22:R658–R663. - PubMed
-
- Buzsáki G. Rhythms of the brain. Oxford University Press; 2006.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

