Ecological validity and clinical utility of Patient-Reported Outcomes Measurement Information System (PROMIS®) instruments for detecting premenstrual symptoms of depression, anger, and fatigue
- PMID: 24630180
- PMCID: PMC4162640
- DOI: 10.1016/j.jpsychores.2014.01.010
Ecological validity and clinical utility of Patient-Reported Outcomes Measurement Information System (PROMIS®) instruments for detecting premenstrual symptoms of depression, anger, and fatigue
Abstract
Objective: This study examined the ecological validity and clinical utility of NIH Patient Reported-Outcomes Measurement Information System (PROMIS®) instruments for anger, depression, and fatigue in women with premenstrual symptoms.
Methods: One-hundred women completed daily diaries and weekly PROMIS assessments over 4weeks. Weekly assessments were administered through Computerized Adaptive Testing (CAT). Weekly CATs and corresponding daily scores were compared to evaluate ecological validity. To test clinical utility, we examined if CATs could detect changes in symptom levels, if these changes mirrored those obtained from daily scores, and if CATs could identify clinically meaningful premenstrual symptom change.
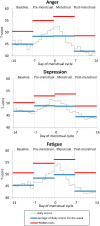
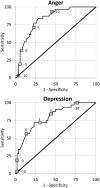
Results: PROMIS CAT scores were higher in the pre-menstrual than the baseline (ps<.0001) and post-menstrual (ps<.0001) weeks. The correlations between CATs and aggregated daily scores ranged from .73 to .88 supporting ecological validity. Mean CAT scores showed systematic changes in accordance with the menstrual cycle and the magnitudes of the changes were similar to those obtained from the daily scores. Finally, Receiver Operating Characteristic (ROC) analyses demonstrated the ability of the CATs to discriminate between women with and without clinically meaningful premenstrual symptom change.
Conclusions: PROMIS CAT instruments for anger, depression, and fatigue demonstrated validity and utility in premenstrual symptom assessment. The results provide encouraging initial evidence of the utility of PROMIS instruments for the measurement of affective premenstrual symptoms.
Keywords: Anger; Depression; Fatigue; PROMIS®; Premenstrual symptoms; Validity.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

References
-
- Halbreich U, Backstrom T, Eriksson E, O'Brien S, Calil H, Ceskova E, Dennerstein L, Douki S, Freeman E, Genazzani A, Heuser I, Kadri N, Rapkin A, Steiner M, Wittchen HU, Yonkers K. Clinical diagnostic criteria for premenstrual syndrome and guidelines for their quantification for research studies. Gynecol Endocrinol. 2007;23:123–30. - PubMed
-
- Halbreich U. The diagnosis of premenstrual syndromes and premenstrual dysphoric disorder--clinical procedures and research perspectives. Gynecol Endocrinol. 2004;19:320–34. - PubMed
-
- Sveindottir H, Backstrom T. Prevalence of menstrual cycle symptom cyclicity and premenstrual dysphoric disorder in a random sample of women using and not using oral contraceptives. Acta Obstet Gynecol Scand. 2000;79:405–13. - PubMed
-
- Yonkers KA, Pearlstein T, Rosenheck RA. Premenstrual disorders: bridging research and clinical reality. Arch Womens Ment Health. 2003;6:287–92. - PubMed
-
- Pearlstein T, Yonkers KA, Fayyad R, Gillespie JA. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord. 2005;85:275–82. - PubMed
Publication types
MeSH terms
Grants and funding
- 1U01AR057948/AR/NIAMS NIH HHS/United States
- U01 AR057940/AR/NIAMS NIH HHS/United States
- U01 AR057948/AR/NIAMS NIH HHS/United States
- U01 AR057956/AR/NIAMS NIH HHS/United States
- U01AR057936/AR/NIAMS NIH HHS/United States
- U01AR052155/AR/NIAMS NIH HHS/United States
- U01 AR057967/AR/NIAMS NIH HHS/United States
- U01 AR052171/AR/NIAMS NIH HHS/United States
- U54 AR057951/AR/NIAMS NIH HHS/United States
- U01AR052170/AR/NIAMS NIH HHS/United States
- U54AR057943/AR/NIAMS NIH HHS/United States
- U01 AR052170/AR/NIAMS NIH HHS/United States
- U01AR052181/AR/NIAMS NIH HHS/United States
- U01AR057940/AR/NIAMS NIH HHS/United States
- U01AR057954/AR/NIAMS NIH HHS/United States
- U01 AR057929/AR/NIAMS NIH HHS/United States
- U01 AR052181/AR/NIAMS NIH HHS/United States
- U54AR057951/AR/NIAMS NIH HHS/United States
- U54AR057926/AR/NIAMS NIH HHS/United States
- 1 U01AR057948-01/AR/NIAMS NIH HHS/United States
- U01 AR057954/AR/NIAMS NIH HHS/United States
- U01AR057948/AR/NIAMS NIH HHS/United States
- U01 AR052177/AR/NIAMS NIH HHS/United States
- U01AR052186/AR/NIAMS NIH HHS/United States
- U01AR052171/AR/NIAMS NIH HHS/United States
- U01 AR052158/AR/NIAMS NIH HHS/United States
- U01 AR057971/AR/NIAMS NIH HHS/United States
- U01AR052158/AR/NIAMS NIH HHS/United States
- U01AR057929/AR/NIAMS NIH HHS/United States
- U01 AR052155/AR/NIAMS NIH HHS/United States
- U01AR057956/AR/NIAMS NIH HHS/United States
- U01AR057967/AR/NIAMS NIH HHS/United States
- U01AR052177/AR/NIAMS NIH HHS/United States
- U54 AR057943/AR/NIAMS NIH HHS/United States
- U01 AR057936/AR/NIAMS NIH HHS/United States
- U01 AR052186/AR/NIAMS NIH HHS/United States
- U01AR057971/AR/NIAMS NIH HHS/United States
- U54 AR057926/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

