VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis
- PMID: 24630601
- PMCID: PMC3969997
- DOI: 10.1016/j.ajpath.2013.12.023
VEGFA activates erythropoietin receptor and enhances VEGFR2-mediated pathological angiogenesis
Abstract
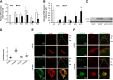
Clinical and animal studies implicate erythropoietin (EPO) and EPO receptor (EPOR) signaling in angiogenesis. In the eye, EPO is involved in both physiological and pathological angiogenesis in the retina. We hypothesized that EPOR signaling is important in pathological angiogenesis and tested this hypothesis using a rat model of oxygen-induced retinopathy that is representative of human retinopathy of prematurity. We first determined that EPOR expression and activation were increased and that activated EPOR was localized to retinal vascular endothelial cells (ECs) in retinas at postnatal day 18 (p18), when pathological angiogenesis in the form of intravitreal neovascularization occurred. In human retinal microvascular ECs, EPOR was up-regulated and activated by VEGF. Lentiviral-delivered shRNAs that knocked down Müller cell-expressed VEGF in the retinopathy of prematurity model also reduced phosphorylated EPOR (p-EPOR) and VEGFR2 (p-VEGFR2) in retinal ECs. In human retinal microvascular ECs, VEGFR2-activated EPOR caused an interaction between p-EPOR and p-VEGFR2; knockdown of EPOR by siRNA transfection reduced VEGF-induced EC proliferation in association with reduced p-VEGFR2 and p-STAT3; however, inhibition of VEGFR2 activation by siRNA transfection or semaxanib (SU5416) abolished VEGFA-induced proliferation of ECs and phosphorylation of VEGFR2, EPOR, and STAT3. Our results show that VEGFA-induced p-VEGFR2 activates EPOR and causes an interaction between p-EPOR and p-VEGFR2 to enhance VEGFA-induced EC proliferation by exacerbating STAT3 activation, leading to pathological angiogenesis.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. - PubMed
-
- Smith L.E.H., Wesolowski E., McLellan A., Kostyk S.K., D’Amato R., Sullivan R., D’Amore P.A. Oxygen induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. - PubMed
-
- Ashton N. Retrolental fibroplasia now retinopathy of prematurity. Br J Ophthalmol. 1984;68:689.
-
- Shah P.K., Narendran V., Kalpana N. Aggressive posterior retinopathy of prematurity in large preterm babies in South India. Arch Dis Child Fetal Neonatal Ed. 2012;97:F371–F375. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

