Caspase-11 controls interleukin-1β release through degradation of TRPC1
- PMID: 24630989
- PMCID: PMC4239700
- DOI: 10.1016/j.celrep.2014.02.015
Caspase-11 controls interleukin-1β release through degradation of TRPC1
Abstract
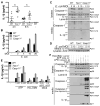
Caspase-11 is a highly inducible caspase that controls both inflammatory responses and cell death. Caspase-11 controls interleukin 1β (IL-1β) secretion by potentiating caspase-1 activation and induces caspase-1-independent pyroptosis downstream of noncanonical NLRP3 inflammasome activators such as lipopolysaccharide (LPS) and Gram-negative bacteria. However, we still know very little about the downstream mechanism of caspase-11 in regulating inflammation because the known substrates of caspase-11 are only other caspases. Here, we identify the cationic channel subunit transient receptor potential channel 1 (TRPC1) as a substrate of caspase-11. TRPC1 deficiency increases the secretion of IL-1β without modulating caspase-1 cleavage or cell death in cultured macrophages. Consistently, trpc1(-/-) mice show higher IL-1β secretion in the sepsis model of intraperitoneal LPS injection. Altogether, our data suggest that caspase-11 modulates the cationic channel composition of the cell and thus regulates the unconventional secretion pathway in a manner independent of caspase-1.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

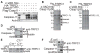
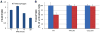
References
-
- Beech DJ. TRPC1: store-operated channel and more. Pflugers Arch. 2005;451:53–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

