Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila
- PMID: 24631243
- PMCID: PMC7613681
- DOI: 10.1016/j.cub.2013.12.051
Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila
Abstract
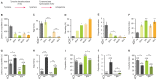
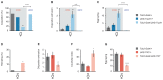
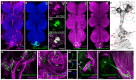
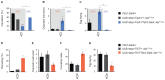
Mating elicits profound behavioral and physiological changes in many species that are crucial for reproductive success. After copulation, Drosophila melanogaster females reduce their sexual receptivity and increase egg laying [1, 2]. Transfer of male sex peptide (SP) during copulation mediates these postmating responses [1, 3-6] via SP sensory neurons in the uterus defined by coexpression of the proprioceptive neuronal marker pickpocket (ppk) and the sex-determination genes doublesex (dsx) and fruitless (fru) [7-9]. Although neurons expressing dsx downstream of SP signaling have been shown to regulate postmating behaviors [9], how the female nervous system coordinates the change from pre- to postcopulatory states is unknown. Here, we show a role of the neuromodulator octopamine (OA) in the female postmating response. Lack of OA disrupts postmating responses in mated females, while increase of OA induces postmating responses in virgin females. Using a novel dsx(FLP) allele, we uncovered dsx neuronal elements associated with OA signaling involved in modulation of postmating responses. We identified a small subset of sexually dimorphic OA/dsx(+) neurons (approximately nine cells in females) in the abdominal ganglion. Our results are consistent with a model whereby OA neuronal signaling increases after copulation, which in turn modulates changes in female behavior and physiology in response to reproductive state.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
Comment in
-
Neural circuits: anatomy of a sexual behavior.Curr Biol. 2014 Apr 14;24(8):R327-9. doi: 10.1016/j.cub.2014.03.009. Curr Biol. 2014. PMID: 24735858
References
-
- Chen PS, Stumm-Zollinger E, Aigaki T, Balmer J, Bienz M, Bohlen P. A male accessory gland peptide that regulates reproductive behavior of female D. melanogaster. Cell. 1988;54:291–298. - PubMed
-
- Aigaki T, Fleischmann I, Chen PS, Kubli E. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron. 1991;7:557–563. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

