Amphiphilic degradable polymers for immobilization and sustained delivery of sphingosine 1-phosphate
- PMID: 24631657
- PMCID: PMC4041830
- DOI: 10.1016/j.actbio.2014.02.051
Amphiphilic degradable polymers for immobilization and sustained delivery of sphingosine 1-phosphate
Abstract
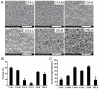
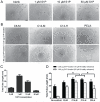
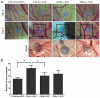
Controlled delivery of the angiogenic factor sphingosine 1-phosphate (S1P) represents a promising strategy for promoting vascularization during tissue repair and regeneration. In this study, we developed an amphiphilic biodegradable polymer platform for the stable encapsulation and sustained release of S1P. Mimicking the interaction between amphiphilic S1P and its binding proteins, a series of polymers with hydrophilic poly(ethylene glycol) core and lipophilic flanking segments of polylactide and/or poly(alkylated lactide) with different alkyl chain lengths were synthesized. These polymers were electrospun into fibrous meshes, and loaded with S1P in generally high loading efficiencies (>90%). Sustained S1P release from these scaffolds could be tuned by adjusting the alkyl chain length, blockiness and lipophilic block length, achieving 35-55% and 45-80% accumulative releases in the first 8h and by 7 days, respectively. Furthermore, using endothelial cell tube formation assay and chicken chorioallantoic membrane assay, we showed that the different S1P loading doses and release kinetics translated into distinct pro-angiogenic outcomes. These results suggest that these amphiphilic polymers are effective delivery vehicles for S1P and may be explored as tissue engineering scaffolds where the delivery of lipophilic or amphiphilic bioactive factors is desired.
Keywords: Amphiphilic copolymer; Angiogenesis; Drug delivery; Sphingosine-1-phosphate; Tissue engineering.
Copyright © 2014 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures



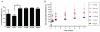
Similar articles
-
Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis.J Biomed Mater Res A. 2018 Jan;106(1):138-146. doi: 10.1002/jbm.a.36217. Epub 2017 Sep 26. J Biomed Mater Res A. 2018. PMID: 28875559 Free PMC article.
-
Sustained delivery of sphingosine-1-phosphate using poly(lactic-co-glycolic acid)-based microparticles stimulates Akt/ERK-eNOS mediated angiogenesis and vascular maturation restoring blood flow in ischemic limbs of mice.Eur J Pharmacol. 2010 May 25;634(1-3):121-31. doi: 10.1016/j.ejphar.2010.02.038. Epub 2010 Mar 3. Eur J Pharmacol. 2010. PMID: 20206620
-
Sustained release of sphingosine 1-phosphate for therapeutic arteriogenesis and bone tissue engineering.Biomaterials. 2008 Jul;29(19):2869-77. doi: 10.1016/j.biomaterials.2008.03.017. Epub 2008 Apr 11. Biomaterials. 2008. PMID: 18405965 Free PMC article.
-
Sphingosine 1-Phosphate (S1P)/ S1P Receptor Signaling and Mechanotransduction: Implications for Intrinsic Tissue Repair/Regeneration.Int J Mol Sci. 2019 Nov 7;20(22):5545. doi: 10.3390/ijms20225545. Int J Mol Sci. 2019. PMID: 31703256 Free PMC article. Review.
-
Novel implications for lysophospholipids, lysophosphatidic acid and sphingosine 1-phosphate, as drug targets in cancer.Anticancer Agents Med Chem. 2009 May;9(4):381-91. doi: 10.2174/1871520610909040381. Anticancer Agents Med Chem. 2009. PMID: 19442039 Review.
Cited by
-
Impaired osteogenesis of T1DM bone marrow-derived stromal cells and periosteum-derived cells and their differential in-vitro responses to growth factor rescue.Stem Cell Res Ther. 2017 Mar 11;8(1):65. doi: 10.1186/s13287-017-0521-6. Stem Cell Res Ther. 2017. PMID: 28283030 Free PMC article.
-
Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis.J Biomed Mater Res A. 2018 Jan;106(1):138-146. doi: 10.1002/jbm.a.36217. Epub 2017 Sep 26. J Biomed Mater Res A. 2018. PMID: 28875559 Free PMC article.
-
Biodegradable PEG-Based Amphiphilic Block Copolymers for Tissue Engineering Applications.ACS Biomater Sci Eng. 2015 Jul 13;1(7):463-480. doi: 10.1021/acsbiomaterials.5b00122. Epub 2015 May 26. ACS Biomater Sci Eng. 2015. PMID: 27175443 Free PMC article.
-
Sphingosine 1-phosphate (S1P) signalling: Role in bone biology and potential therapeutic target for bone repair.Pharmacol Res. 2017 Nov;125(Pt B):232-245. doi: 10.1016/j.phrs.2017.08.013. Epub 2017 Sep 22. Pharmacol Res. 2017. PMID: 28855094 Free PMC article. Review.
-
Synthesis and characterization of 3-methyl-6-[(propyn-yloxy)meth-yl]-1,4-dioxane-2,5-dione.Acta Crystallogr E Crystallogr Commun. 2017 Jun 16;73(Pt 7):1044-1047. doi: 10.1107/S2056989017008581. eCollection 2017 Jul 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28775879 Free PMC article.
References
-
- Carano RA, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–9. - PubMed
-
- Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, et al. Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng. 2006;12:2093–104. - PubMed
-
- Harris GM, Rutledge K, Cheng Q, Blanchette J, Jabbarzadeh E. Strategies to direct angiogenesis within scaffolds for bone tissue engineering. Curr Pharm Des. 2013;19:3456–65. - PubMed
-
- Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63:300–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

