Systems analysis of auxin transport in the Arabidopsis root apex
- PMID: 24632533
- PMCID: PMC4001398
- DOI: 10.1105/tpc.113.119495
Systems analysis of auxin transport in the Arabidopsis root apex
Abstract
Auxin is a key regulator of plant growth and development. Within the root tip, auxin distribution plays a crucial role specifying developmental zones and coordinating tropic responses. Determining how the organ-scale auxin pattern is regulated at the cellular scale is essential to understanding how these processes are controlled. In this study, we developed an auxin transport model based on actual root cell geometries and carrier subcellular localizations. We tested model predictions using the DII-VENUS auxin sensor in conjunction with state-of-the-art segmentation tools. Our study revealed that auxin efflux carriers alone cannot create the pattern of auxin distribution at the root tip and that AUX1/LAX influx carriers are also required. We observed that AUX1 in lateral root cap (LRC) and elongating epidermal cells greatly enhance auxin's shootward flux, with this flux being predominantly through the LRC, entering the epidermal cells only as they enter the elongation zone. We conclude that the nonpolar AUX1/LAX influx carriers control which tissues have high auxin levels, whereas the polar PIN carriers control the direction of auxin transport within these tissues.
Figures
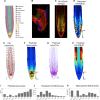





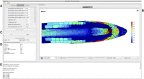
References
-
- Abas L., Benjamins R., Malenica N., Paciorek T., Wiśniewska J., Moulinier-Anzola J.C., Sieberer T., Friml J., Luschnig C. (2006). Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 8: 249–256. Erratum. Nat. Cell Biol. 8: 424. - PubMed
-
- Band L.R., King J.R. (2012). Multiscale modelling of auxin transport in the plant-root elongation zone. J. Math. Biol. 65: 743–785. - PubMed
-
- Benjamins R., Scheres B. (2008). Auxin: The looping star in plant development. Annu. Rev. Plant Biol. 59: 443–465. - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

