Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound
- PMID: 24632555
- PMCID: PMC3968378
- DOI: 10.3390/toxins6031080
Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound
Abstract
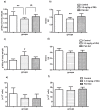
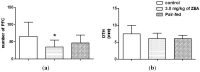
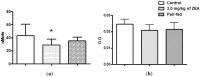
The aim of this study was to assess the toxic effects of zearalenone (ZEA) on the immune function. Ovariectomised rats were treated daily by gavage with 3.0 mg/kg of ZEA for 28 days. Body weight gain, food consumption, haemotological parameters, lymphoid organs, and their cellularities were evaluated. Moreover, acquired immune responses and macrophage activity were also assessed. ZEA promoted reduction in body weight gain, which is not fully explained by diminished food consumption. Despite no effect on haematological parameters, ZEA caused thymic atrophy with histological and thymocyte phenotype changes and decrease in the B cell percentage in the spleen. With respect to acquired and innate immune responses, no statistically significant differences in delayed-type hypersensitivity were noticed; however, in the ZEA-treated rats, antibody production and peroxide release by macrophages were impaired. The observed results could be related to ZEA activity on ERs; thus, ZEA is an immunotoxic compound similar to estrogen and some endocrine disruptors.
Figures

References
-
- Hestbjerg H., Nielsen K.F., Thrane U., Elmholt S. Production of trichothecenes and other secondary metabolites by Fusarium culmorum and Fusarium equiseti on common laboratory media and a soil organic matter agar: An ecological interpretation. J. Agric. Food Chem. 2002;50:7593–7599. doi: 10.1021/jf020432o. - DOI - PubMed
-
- Collection of Occurrence Data of Fusarium Toxins in Food Andassessment of Dietary Intake by the Population of EU Member States; SCOOP European Project, task 3.2.10. 2003. [(accessed on 17 October 2013)]. Available online: http://ec.europa.eu/food/fs/scoop/task3210.pdf. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

