Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis
- PMID: 24632637
- PMCID: PMC3954806
- DOI: 10.1371/journal.pone.0091848
Deregulation of mitochondria-shaping proteins Opa-1 and Drp-1 in manganese-induced apoptosis
Abstract
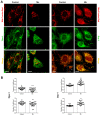
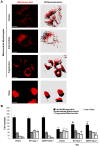
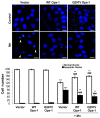
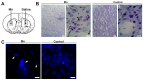
Mitochondria are dynamic organelles that undergo fusion and fission processes. These events are regulated by mitochondria-shaping proteins. Changes in the expression and/or localization of these proteins lead to a mitochondrial dynamics impairment and may promote apoptosis. Increasing evidence correlates the mitochondrial dynamics disruption with the occurrence of neurodegenerative diseases. Therefore, we focused on this topic in Manganese (Mn)-induced Parkinsonism, a disorder associated with Mn accumulation preferentially in the basal ganglia where mitochondria from astrocytes represent an early target. Using MitoTracker Red staining we observed increased mitochondrial network fission in Mn-exposed rat astrocytoma C6 cells. Moreover, Mn induced a marked decrease in fusion protein Opa-1 levels as well as a dramatic increase in the expression of fission protein Drp-1. Additionally, Mn provoked a significant release of high MW Opa-1 isoforms from the mitochondria to the cytosol as well as an increased Drp-1 translocation to the mitochondria. Both Mdivi-1, a pharmacological Drp-1 inhibitor, and rat Drp-1 siRNA reduced the number of apoptotic nuclei, preserved the mitochondrial network integrity and prevented cell death. CsA, an MPTP opening inhibitor, prevented mitochondrial Δψm disruption, Opa-1 processing and Drp-1 translocation to the mitochondria therefore protecting Mn-exposed cells from mitochondrial disruption and apoptosis. The histological analysis and Hoechst 33258 staining of brain sections of Mn-injected rats in the striatum showed a decrease in cellular mass paralleled with an increase in the occurrence of apoptotic nuclei. Opa-1 and Drp-1 expression levels were also changed by Mn-treatment. Our results demonstrate for the first time that abnormal mitochondrial dynamics is implicated in both in vitro and in vivo Mn toxicity. In addition we show that the imbalance in fusion/fission equilibrium might be involved in Mn-induced apoptosis. This knowledge may provide new therapeutic tools for the treatment of Manganism and other neurodegenerative diseases.
Conflict of interest statement
Figures






References
-
- Couper J (1837) On the effects of black oxide of manganese when inhaled in the lungs. Br Ann Med Pharmacol 1: 41–42.
-
- Roth JA (2009) Are there common biochemical and molecular mechanisms controlling manganism and parkisonism. Neuromolecular Med 11: 281–96. - PubMed
-
- Benedetto A, Au C, Aschner M (2009) Manganese-Induced Dopaminergic Neurodegeneration: Insights into Mechanisms and Genetics Shared with Parkinson Disease. Chem Rev 109: 4862–4884. - PubMed
-
- Olanow CW (2004) Manganese-induced parkinsonism and Parkinson’s disease. Ann NY Acad Sci 1012: 209–23. - PubMed
-
- Zhao F, Cai T, Liu M, Zheng G, Luo W, et al. (2009) Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci 107: 156–64. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

