Multiple roles of the coagulation protease cascade during virus infection
- PMID: 24632711
- PMCID: PMC3999750
- DOI: 10.1182/blood-2013-09-526277
Multiple roles of the coagulation protease cascade during virus infection
Abstract
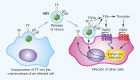
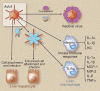
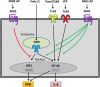
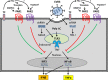
The coagulation cascade is activated during viral infections. This response may be part of the host defense system to limit spread of the pathogen. However, excessive activation of the coagulation cascade can be deleterious. In fact, inhibition of the tissue factor/factor VIIa complex reduced mortality in a monkey model of Ebola hemorrhagic fever. Other studies showed that incorporation of tissue factor into the envelope of herpes simplex virus increases infection of endothelial cells and mice. Furthermore, binding of factor X to adenovirus serotype 5 enhances infection of hepatocytes but also increases the activation of the innate immune response to the virus. Coagulation proteases activate protease-activated receptors (PARs). Interestingly, we and others found that PAR1 and PAR2 modulate the immune response to viral infection. For instance, PAR1 positively regulates TLR3-dependent expression of the antiviral protein interferon β, whereas PAR2 negatively regulates expression during coxsackievirus group B infection. These studies indicate that the coagulation cascade plays multiple roles during viral infections.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

