Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study
- PMID: 24632748
- PMCID: PMC3954629
- DOI: 10.1371/journal.pone.0091293
Efficacy of combination therapy with oseltamivir phosphate and azithromycin for influenza: a multicenter, open-label, randomized study
Abstract
Background: Macrolides have antibiotic and immunomodulatory activities, which may have a favorable effect on the clinical outcome of patients with infections, including influenza. This study aimed to evaluate the effects of combination therapy with an anti-influenza agent, oseltamivir, and a single-dose formulation of azithromycin (AZM), which has been used for influenza-related secondary pneumonia, on influenza patients. The primary endpoint was a change in the expression levels of inflammatory cytokines. Secondary endpoints were the time required for resolution of influenza-related symptoms, incidence of complications, and adverse reactions.
Methods: Patients with seasonal influenza were enrolled in this multicenter, open-label, randomized study. Patients were stratified according to the presence of a high risk factor and were randomized to receive combination therapy with oseltamivir plus an extended-release formulation of AZM (combo-group) or oseltamivir monotherapy (mono-group).
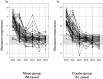
Results: We enrolled 107 patients and randomized them into the mono-group (56 patients) or the combo-group (51 patients). All patients were diagnosed with influenza A infection, and none of the patients had comorbid pneumonia. Statistically significant differences were not observed in the expression levels of inflammatory cytokines and chemokines between the 2 groups. The maximum temperature in the combo-group was lower than that in the mono-group on day 3 through day 5 (p = 0.048), particularly on day 4 (p = 0.037).
Conclusion: To our knowledge, this is the first prospective, randomized, clinical trial of oseltamivir and AZM combination therapy for influenza. Although the difference in inflammatory cytokine expression level was not statistically significant, combination therapy showed an early resolution of some symptoms.
Name of registry: University hospital Medical Information Network (UMIN).
Trial registration no: UMIN000005371.
Conflict of interest statement
Figures


References
-
- Woodhead M, Blasi F, Ewig S, Huchon G, Ieven M, et al. (2005) Guidelines for the management of adult lower respiratory tract infections. Eur Respir J 26: 1138–1180. - PubMed
-
- Kosai K, Seki M, Yanagihara K, Nakamura S, Kurihara S, et al. (2008) Elevated levels of high mobility group box chromosomal protein-1 (HMGB-1) in sera from patients with severe bacterial pneumonia coinfected with influenza virus. Scand J Infect Dis 40: 338–342. - PubMed
-
- Konstantinos AP, Sheridan JF (2001) Stress and influenza viral infection: modulation of proinflammatory cytokine responses in the lung. Respir Physiol 128: 71–77. - PubMed
-
- Deng R, Lu M, Korteweg C, Gao Z, McNutt MA, et al. (2008) Distinctly different expression of cytokines and chemokines in the lungs of two H5N1 avian influenza patients. J Pathol 216: 328–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

