Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation
- PMID: 24632837
- PMCID: PMC3954790
- DOI: 10.1371/journal.pone.0091675
Skeletal muscle perilipin 3 and coatomer proteins are increased following exercise and are associated with fat oxidation
Abstract
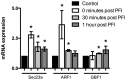
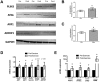
Lipid droplet-associated proteins such as perilipin 3 (PLIN3) and coatomer GTPase proteins (GBF1, ARF1, Sec23a, and ARFRP1) are expressed in skeletal muscle but little is known so far as to their regulation of lipolysis. We aimed here to explore the effects of lipolytic stimulation in vitro in primary human myotubes as well as in vivo following an acute exercise bout. In vitro lipolytic stimulation by epinephrine (100 μM) or by a lipolytic cocktail (30 μM palmitate, 4 μM forskolin, and 0.5 μM ionomycin, PFI) resulted in increases in PLIN3 protein content. Coatomer GTPases such as GBF1, ARF1, Sec23a, and ARFRP1 also increased in response to lipolytic stimuli. Furthermore, a long duration endurance exercise bout (20 males; age 24.0 ± 4.5 y; BMI 23.6 ± 1.8 kg/m(2)) increased PLIN3 protein in human skeletal muscle (p = 0.03) in proportion to ex vivo palmitate oxidation (r = 0.45, p = 0.04) and whole body in vivo fat oxidation (r = 0.52, p = 0.03). Protein content of ARF1 was increased (p = 0.04) while mRNA expression was increased for several other coatomers (GBF1, ARF1, and Sec23a, all p<0.05). These data provide novel observational insight into the possible relationships between lipolysis and PLIN3 along with these coatomoer GTPase proteins in human skeletal muscle.
Conflict of interest statement
Figures


References
-
- Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, et al. (1999) Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42: 113–116. - PubMed
-
- Jacob S, Machann J, Rett K, Brechtel K, Volk A, et al. (1999) Association of increased intramyocellular lipid content with insulin resistance in lean nondiabetic offspring of type 2 diabetic subjects. Diabetes 48: 1113–1119. - PubMed
-
- Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, et al. (2002) Assessment of skeletal muscle triglyceride content by (1)H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51: 1022–1027. - PubMed
-
- Goodpaster BH, He J, Watkins S, Kelley DE (2001) Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab 86: 5755–5761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

