MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes
- PMID: 24633305
- PMCID: PMC3981675
- DOI: 10.1038/nm.3482
MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes
Abstract
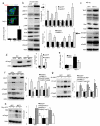
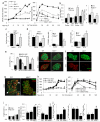
Apoptotic cell death is a hallmark of the loss of insulin-producing beta cells in all forms of diabetes mellitus. Current treatments fail to halt the decline in functional beta cell mass, and strategies to prevent beta cell apoptosis and dysfunction are urgently needed. Here, we identified mammalian sterile 20-like kinase-1 (MST1) as a critical regulator of apoptotic beta cell death and function. Under diabetogenic conditions, MST1 was strongly activated in beta cells in human and mouse islets and specifically induced the mitochondrial-dependent pathway of apoptosis through upregulation of the BCL-2 homology-3 (BH3)-only protein BIM. MST1 directly phosphorylated the beta cell transcription factor PDX1 at T11, resulting in the latter's ubiquitination and degradation and thus in impaired insulin secretion. MST1 deficiency completely restored normoglycemia, beta cell function and survival in vitro and in vivo. We show MST1 as a proapoptotic kinase and key mediator of apoptotic signaling and beta cell dysfunction and suggest that it may serve as target for the development of new therapies for diabetes.
Figures





Comment in
-
Target identification: Stopping β-cell death in diabetes.Nat Rev Drug Discov. 2014 May;13(5):333. doi: 10.1038/nrd4313. Epub 2014 Apr 11. Nat Rev Drug Discov. 2014. PMID: 24722464 No abstract available.
References
-
- Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414:792–798. - PubMed
-
- Butler AE, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. - PubMed
-
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. - PubMed
-
- Donath MY, Storling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J.Mol.Med. 2003;81:455–470. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

