Mechanical memory and dosing influence stem cell fate
- PMID: 24633344
- PMCID: PMC4031270
- DOI: 10.1038/nmat3889
Mechanical memory and dosing influence stem cell fate
Abstract
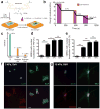
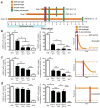
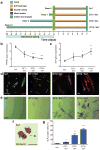
We investigated whether stem cells remember past physical signals and whether these can be exploited to dose cells mechanically. We found that the activation of the Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding domain (TAZ) as well as the pre-osteogenic transcription factor RUNX2 in human mesenchymal stem cells (hMSCs) cultured on soft poly(ethylene glycol) (PEG) hydrogels (Young's modulus E ~ 2 kPa) depended on previous culture time on stiff tissue culture polystyrene (TCPS; E ~ 3 GPa). In addition, mechanical dosing of hMSCs cultured on initially stiff (E ~ 10 kPa) and then soft (E ~ 2 kPa) phototunable PEG hydrogels resulted in either reversible or--above a threshold mechanical dose--irreversible activation of YAP/TAZ and RUNX2. We also found that increased mechanical dosing on supraphysiologically stiff TCPS biases hMSCs towards osteogenic differentiation. We conclude that stem cells possess mechanical memory--with YAP/TAZ acting as an intracellular mechanical rheostat--that stores information from past physical environments and influences the cells' fate.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

Comment in
-
Stem cell differentiation: sticky mechanical memory.Nat Mater. 2014 Jun;13(6):542-3. doi: 10.1038/nmat3989. Nat Mater. 2014. PMID: 24845990 No abstract available.
References
-
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and rhoa regulate stem cell lineage commitment. Developmental Cell. 2004;6:483–495. - PubMed
-
- McMurray RJ, et al. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat Mater. 2011;10:637–644. - PubMed
-
- Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and longterm cellular responses to dynamic mechanics. Nature Communications. 2012;3 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

