Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway
- PMID: 24634501
- PMCID: PMC4031946
- DOI: 10.1194/jlr.M047357
Dietary omega-3 fatty acids modulate the eicosanoid profile in man primarily via the CYP-epoxygenase pathway
Abstract
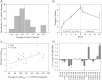
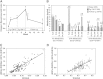
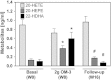
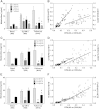
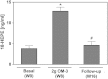
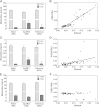
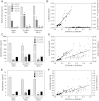
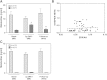
Cytochrome P450 (CYP)-dependent metabolites of arachidonic acid (AA) contribute to the regulation of cardiovascular function. CYP enzymes also accept EPA and DHA to yield more potent vasodilatory and potentially anti-arrhythmic metabolites, suggesting that the endogenous CYP-eicosanoid profile can be favorably shifted by dietary omega-3 fatty acids. To test this hypothesis, 20 healthy volunteers were treated with an EPA/DHA supplement and analyzed for concomitant changes in the circulatory and urinary levels of AA-, EPA-, and DHA-derived metabolites produced by the cyclooxygenase-, lipoxygenase (LOX)-, and CYP-dependent pathways. Raising the Omega-3 Index from about four to eight primarily resulted in a large increase of EPA-derived CYP-dependent epoxy-metabolites followed by increases of EPA- and DHA-derived LOX-dependent monohydroxy-metabolites including the precursors of the resolvin E and D families; resolvins themselves were not detected. The metabolite/precursor fatty acid ratios indicated that CYP epoxygenases metabolized EPA with an 8.6-fold higher efficiency and DHA with a 2.2-fold higher efficiency than AA. Effects on leukotriene, prostaglandin E, prostacyclin, and thromboxane formation remained rather weak. We propose that CYP-dependent epoxy-metabolites of EPA and DHA may function as mediators of the vasodilatory and cardioprotective effects of omega-3 fatty acids and could serve as biomarkers in clinical studies investigating the cardiovascular effects of EPA/DHA supplementation.
Keywords: cytochrome P450; lipidomics; nutrition.
Copyright © 2014 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Konkel A., Schunck W. H. 2011. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta. 1814: 210–222. - PubMed
-
- Capdevila J. H., Falck J. R. 2002. Biochemical and molecular properties of the cytochrome P450 arachidonic acid monooxygenases. Prostaglandins Other Lipid Mediat. 68–69: 325–344. - PubMed
-
- Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875. - PubMed
-
- Kroetz D. L., Zeldin D. C. 2002. Cytochrome P450 pathways of arachidonic acid metabolism. Curr. Opin. Lipidol. 13: 273–283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

