The Impact of Enzyme Orientation and Electrode Topology on the Catalytic Activity of Adsorbed Redox Enzymes
- PMID: 24634538
- PMCID: PMC3951721
- DOI: 10.1016/j.electacta.2013.01.153
The Impact of Enzyme Orientation and Electrode Topology on the Catalytic Activity of Adsorbed Redox Enzymes
Abstract
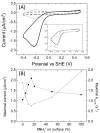
It is well established that the structural details of electrodes and their interaction with adsorbed enzyme influences the interfacial electron transfer rate. However, for nanostructured electrodes, it is likely that the structure also impacts on substrate flux near the adsorbed enzymes and thus catalytic activity. Furthermore, for enzymes converting macro-molecular substrates it is possible that the enzyme orientation determines the nature of interactions between the adsorbed enzyme and substrate and therefore catalytic rates. In essence the electrode may impede substrate access to the active site of the enzyme. We have tested these possibilities through studies of the catalytic performance of two enzymes adsorbed on topologically distinct electrode materials. Escherichia coli NrfA, a nitrite reductase, was adsorbed on mesoporous, nanocrystalline SnO2 electrodes. CymA from Shewanella oneidensis MR-1 reduces menaquinone-7 within 200 nm sized liposomes and this reaction was studied with the enzyme adsorbed on SAM modified ultra-flat gold electrodes.
Keywords: Self-assembled monolayer (SAM); cytochrome; lipid vesicle; protein-film electrochemistry (PFE); quinone.
Figures






References
-
- Leger C, Bertrand P. Chem. Rev. 2008;108:2379–2438. - PubMed
-
- Armstrong FA. Curr. Opin. Chem. Biol. 2005;9:110–117. - PubMed
-
- Zhang JD, Kuznetsov AM, Medvedev IG, Chi QJ, Albrecht T, Jensen PS, Ulstrup J. Chem. Rev. 2008;108:2737–2791. - PubMed
-
- Cracknell JA, Vincent KA, Armstrong FA. Chem. Rev. 2008;108:2439–2461. - PubMed
-
- Willner I, Yan YM, Willner B, Tel-Vered R. Fuel Cells. 2009;9:7–24.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
