Direct role of interrod spacing in mediating cell adhesion on Sr-HA nanorod-patterned coatings
- PMID: 24634585
- PMCID: PMC3952902
- DOI: 10.2147/IJN.S58236
Direct role of interrod spacing in mediating cell adhesion on Sr-HA nanorod-patterned coatings
Abstract
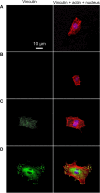
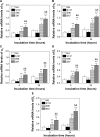
The process in which nanostructured surfaces mediate cell adhesion is not well understood, and may be indirect (via protein adsorption) or direct. We prepared Sr-doped hydroxyapatite (Sr1-HA) 3D nanorods (with interrod spacing of 67.3 ± 3.8, 95.7 ± 4.2, and 136.8 ± 8.7 nm) and 2D nanogranulate patterned coatings on titanium. Employing the coatings under the same surface chemistry and roughness, we investigated the indirect/direct role of Sr1-HA nanotopographies in the regulation of osteoblast adhesion in both serum-free and serum-containing Dulbecco's Modified Eagle/Ham's Medium. The results reveal that the number of adherent cells, cell-secreted anchoring proteins (fibronectin, vitronectin, and collagen), vinculin and focal adhesion kinase (FAK) denoted focal adhesion (FA) contact, and gene expression of vinculin, FAK, and integrin subunits (α2, α5, αv, β1, and β3), undergo significant changes in the inter-nanorod spacing and dimensionality of Sr1-HA nanotopographies in the absence of serum; they are significantly enhanced on the <96 nm spaced nanorods and more pronounced with decreasing interrod spacing. However, they are inhibited on the >96 nm spaced nanorods compared to nanogranulated 2D topography. Although the adsorption of fibronectin and vitronectin from serum are higher on 136.8 ± 8.7 nm spaced nanorod patterned topography than nanogranulated topography, cell adhesion is inhibited on the former compared to the latter in the presence of serum, further suggesting that reduced cell adhesion is independent of protein adsorption. It is clearly indicated that 3D nanotopography can directly modulate cell adhesion by regulating integrins, which subsequently mediate anchoring proteins' secretion and FA formation rather than via protein adsorption.
Keywords: anchoring protein secretion; focal adhesion; integrin; inter-nanorod spacing; nanotopography; osteoblast adhesion.
Figures






References
-
- Anselme K. Osteoblast adhesion on biomaterials. Biomaterials. 2000;21(7):667–681. - PubMed
-
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33. - PubMed
-
- Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol. 2001;2(11):793–805. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

