Comparison of the dynamics of neural interactions between current-based and conductance-based integrate-and-fire recurrent networks
- PMID: 24634645
- PMCID: PMC3943173
- DOI: 10.3389/fncir.2014.00012
Comparison of the dynamics of neural interactions between current-based and conductance-based integrate-and-fire recurrent networks
Abstract
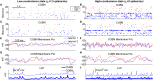
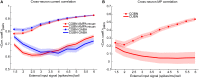
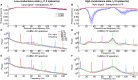
Models of networks of Leaky Integrate-and-Fire (LIF) neurons are a widely used tool for theoretical investigations of brain function. These models have been used both with current- and conductance-based synapses. However, the differences in the dynamics expressed by these two approaches have been so far mainly studied at the single neuron level. To investigate how these synaptic models affect network activity, we compared the single neuron and neural population dynamics of conductance-based networks (COBNs) and current-based networks (CUBNs) of LIF neurons. These networks were endowed with sparse excitatory and inhibitory recurrent connections, and were tested in conditions including both low- and high-conductance states. We developed a novel procedure to obtain comparable networks by properly tuning the synaptic parameters not shared by the models. The so defined comparable networks displayed an excellent and robust match of first order statistics (average single neuron firing rates and average frequency spectrum of network activity). However, these comparable networks showed profound differences in the second order statistics of neural population interactions and in the modulation of these properties by external inputs. The correlation between inhibitory and excitatory synaptic currents and the cross-neuron correlation between synaptic inputs, membrane potentials and spike trains were stronger and more stimulus-modulated in the COBN. Because of these properties, the spike train correlation carried more information about the strength of the input in the COBN, although the firing rates were equally informative in both network models. Moreover, the network activity of COBN showed stronger synchronization in the gamma band, and spectral information about the input higher and spread over a broader range of frequencies. These results suggest that the second order statistics of network dynamics depend strongly on the choice of synaptic model.
Keywords: conductance based neuron models; correlation analysis; current based neuron models; information encoding; integrate-and-fire neurons; local field potentials; recurrent neural network; spike correlation.
Figures









References
-
- Berens P. (2009). CircStat: a MATLAB toolbox for circular statistics. J. Stat. Softw. 31, 1–21
-
- Braitenberg V., SchüZ A. (1991). Anatomy of the Cortex: Statistics and Geometry. Berlin; New York, NY: Springer-Verlag; 10.1007/978-3-662-02728-8 - DOI
-
- Brunel N. (2013). Dynamics of neural networks, in Principles of Neural Coding, eds Quian Quiroga R., Panzeri S. (Boca Raton, FL: CRC Press; ), 489–512 10.1201/b14756-29 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

