Wistar rats: a forgotten model of age-related hearing loss
- PMID: 24634657
- PMCID: PMC3942650
- DOI: 10.3389/fnagi.2014.00029
Wistar rats: a forgotten model of age-related hearing loss
Abstract
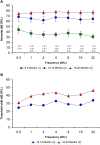
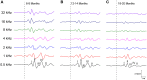
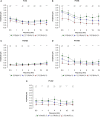
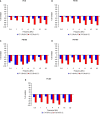
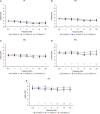
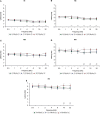
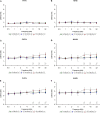
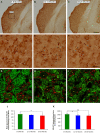
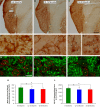
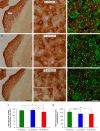
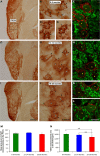
Age-related hearing loss (ARHL) is one of the most frequent sensory impairments in senescence and is a source of important socio-economic consequences. Understanding the pathological responses that occur in the central auditory pathway of patients who suffer from this disability is vital to improve its diagnosis and treatment. Therefore, the goal of this study was to characterize age-related modifications in auditory brainstem responses (ABR) and to determine whether these functional responses might be accompanied by an imbalance between excitation and inhibition in the cochlear nucleus of Wistar rats. To do so, ABR recordings at different frequencies and immunohistochemistry for the vesicular glutamate transporter 1 (VGLUT1) and the vesicular GABA transporter (VGAT) in the ventral cochlear nucleus (VCN) were performed in young, middle-aged and old male Wistar rats. The results demonstrate that there was a significant increase in the auditory thresholds, a significant decrease in the amplitudes and an increase in the latencies of the ABR waves as the age of the rat increased. Additionally, there were decreases in VGLUT1 and VGAT immunostaining in the VCN of older rats compared to younger rats. Therefore, the observed age-related decline in the magnitude of auditory evoked responses might be due in part to a reduction in markers of excitatory function; meanwhile, the concomitant reduction in both excitatory and inhibitory markers might reflect a common central alteration in animal models of ARLH. Together, these findings highlight the suitability of the Wistar rat as an excellent model to study ARHL.
Keywords: auditory brainstem response (ABR); cochlear nucleus; hearing loss; presbyacusis; rat model; vesicular transport proteins.
Figures
References
-
- Alvarado J. C., Fuentes-Santamaria V., Franklin S. R., Brunso-Bechtold J. K., Henkel C. K. (2005). Unilateral cochlear ablation in adult ferrets results in upregulation in calretinin immunostaining in the central nucleus of the inferior colliculus. Neuroscience 136, 957–969 10.1016/j.neuroscience.2005.04.022 - DOI - PubMed
-
- Alvarado J. C., Fuentes-Santamaria V., Franklin S. R., Brunso-Bechtold J. K., Henkel C. K. (2007). Synaptophysin and insulin-like growth factor-1 immunostaining in the central nucleus of the inferior colliculus in adult ferrets following unilateral cochlear removal: a densitometric analysis. Synapse 61, 288–302 10.1002/syn.20373 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

