Mitoribosomal regulation of OXPHOS biogenesis in plants
- PMID: 24634672
- PMCID: PMC3942809
- DOI: 10.3389/fpls.2014.00079
Mitoribosomal regulation of OXPHOS biogenesis in plants
Abstract
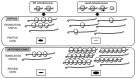
The ribosome filter hypothesis posits that ribosomes are not simple non-selective translation machines but may also function as regulatory elements in protein synthesis. Recent data supporting ribosomal filtering come from plant mitochondria where it has been shown that translation of mitochondrial transcripts encoding components of oxidative phosphorylation complexes (OXPHOS) and of mitoribosomes can be differentially affected by alterations in mitoribosomes. The biogenesis of mitoribosome was perturbed by silencing of a gene encoding a small-subunit protein of the mitoribosome in Arabidopsis thaliana. As a consequence, the mitochondrial OXPHOS and ribosomal transcripts were both upregulated, but only the ribosomal proteins were oversynthesized, while the OXPHOS subunits were actually depleted. This finding implies that the heterogeneity of plant mitoribosomes found in vivo could contribute to the functional selectivity of translation under distinct conditions. Furthermore, global analysis indicates that biogenesis of OXPHOS complexes in plants can be regulated at different levels of mitochondrial and nuclear gene expression, however, the ultimate coordination of genome expression occurs at the complex assembly level.
Keywords: OXPHOS; coordination of gene expression; mitochondrial translation; mitoribosome; ribosome filter hypothesis; ribosome heterogeneity.
Figures
References
-
- Bonen L. (2004). “Translational machinery in plant organelles,” in Molecular Biology and Biotechnology of Plant Organelles eds Daniell H., Chase C. (Netherlands: Springer) 323–345 10.1007/978-1-4020-3166-3_12 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

