Inteins: Nature's Gift to Protein Chemists
- PMID: 24634716
- PMCID: PMC3949740
- DOI: 10.1039/C3SC52951G
Inteins: Nature's Gift to Protein Chemists
Abstract
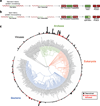
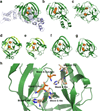
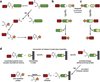
Inteins are auto-processing domains found in organisms from all domains of life. These proteins carry out a process known as protein splicing, which is a multi-step biochemical reaction comprised of both the cleavage and formation of peptide bonds. While the endogenous substrates of protein splicing are specific essential proteins found in intein-containing host organisms, inteins are also functional in exogenous contexts and can be used to chemically manipulate virtually any polypeptide backbone. Given this, protein chemists have exploited various facets of intein reactivity to modify proteins in myriad ways for both basic biological research as well as potential therapeutic applications. Here, we review the intein field, first focusing on the biological context and phylogenetic diversity of inteins, followed by a description of intein structure and biochemical function. Finally, we discuss prevalent inteinbased technologies, focusing on their applications in chemical biology, followed by persistent caveats of intein chemistry and approaches to alleviate these shortcomings. The findings summarized herein describe two and a half decades of research, leading from a biochemical curiosity to the development of powerful protein engineering tools.
Figures



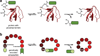
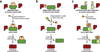
References
-
- Anfinsen CB. Science. 1973;181:223–230. - PubMed
-
- Hartl FU, Hayer-Hartl M. Nat. Struct. Mol. Biol. 2009;16:574–581. - PubMed
-
- Uversky VN, Gillespie JR, Fink AL. Protein Struct. Funct. Genet. 2000;41:415–427. - PubMed
-
- Walsh CT, Garneau-Tsodikova S, Gatto GJ. Angew. Chem. Int. Ed. 2005;44:7342–7372. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

