Transsulfuration Is a Significant Source of Sulfur for Glutathione Production in Human Mammary Epithelial Cells
- PMID: 24634789
- PMCID: PMC3949734
- DOI: 10.1155/2013/637897
Transsulfuration Is a Significant Source of Sulfur for Glutathione Production in Human Mammary Epithelial Cells
Abstract
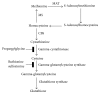
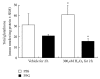
The transsulfuration pathway, through which homocysteine from the methionine cycle provides sulfur for cystathionine formation, which may subsequently be used for glutathione synthesis, has not heretofore been identified as active in mammary cells. Primary human mammary epithelial cells (HMEC's) were labeled with 35S-methionine for 24 hours following pretreatment with a vehicle control, the cysteine biosynthesis inhibitor propargylglycine or the gamma-glutamylcysteine synthesis inhibitor buthionine sulfoximine. Cell lysates were prepared and reacted with glutathione-S-transferase and the fluorescent labeling compound monochlorobimane to form a fluorescent glutathione-bimane conjugate. Comparison of fluorographic and autoradiographic images indicated that glutathione had incorporated 35S-methionine demonstrating that functional transsulfuration occurs in mammary cells. Pathway inhibitors reduced incorporation by roughly 80%. Measurement of glutathione production in HMEC's treated with and without hydrogen peroxide and/or pathway inhibitors indicates that the transsulfuration pathway plays a significant role in providing cysteine for glutathione production both normally and under conditions of oxidant stress.
Figures

References
-
- Lu S. C., Martinez-Chantar M. L., Mato J. M. Methionine adenosyltransferase and S-adenosylmethionine in alcoholic liver disease. Journal of Gastroenterology and Hepatology. 2006;21(supplement 3):S61–S64. - PubMed
-
- Banerjee R., Evande R., Kabil O., Ojha S., Taoka S. Reaction mechanism and regulation of cystathionine beta-synthase. Biochimica et Biophysica Acta. 2003;1647(1-2):30–35. - PubMed
-
- Jill James S., Melnyk S., Jernigan S., Hubanks A., Rose S., Gaylor D. W. Abnormal transmethylation/transsulfuration metabolism and DNA hypomethylation among parents of children with autism. Journal of Autism and Developmental Disorders. 2008;38(10):1966–1975. doi: 10.1007/s10803-008-0591-5. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
