P450-catalyzed intramolecular sp3 C-H amination with arylsulfonyl azide substrates
- PMID: 24634794
- PMCID: PMC3949735
- DOI: 10.1021/cs400893n
P450-catalyzed intramolecular sp3 C-H amination with arylsulfonyl azide substrates
Abstract
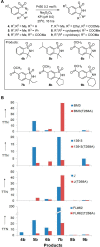
The direct amination of aliphatic C-H bonds represents a most valuable transformation in organic chemistry. While a number of transition metal-based catalysts have been developed and investigated for this purpose, the possibility to execute this transformation with biological catalysts has remained largely unexplored. Here, we report that cytochrome P450 enzymes can serve as efficient catalysts for mediating intramolecular benzylic C-H amination reactions in a variety of arylsulfonyl azide compouds. Under optimized conditions, the P450 catalysts were found to support up to 390 total turnovers leading to the formation of the desired sultam products with excellent regioselectivity. In addition, the chiral environment provided by the enzyme active site allowed for the reaction to proceed in a stereo- and enantioselective manner. The C-H amination activity, substrate profile, and enantio/stereoselectivity of these catalysts could be modulated by utilizing enzyme variants with engineered active sites.
Keywords: C—H amination; arylsulfonyl azides; cytochrome P450; enzymatic catalysis; protein engineering; sultams.
Figures


References
-
- Muller P.; Fruit C. Chem. Rev. 2003, 10382905–2919. - PubMed
-
- Collet F.; Dodd R. H.; Dauban P. Chem. Commun. 2009, 34, 5061–5074. - PubMed
-
- Espino C. G.; Du Bois J. Angew. Chem., Int. Ed. 2001, 403598–600. - PubMed
-
- Fiori K. W.; Fleming J. J.; Du Bois J. Angew. Chem., Int. Ed. 2004, 43334349–4352. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
