Hippocampal lipoprotein lipase regulates energy balance in rodents
- PMID: 24634821
- PMCID: PMC3953702
- DOI: 10.1016/j.molmet.2013.11.002
Hippocampal lipoprotein lipase regulates energy balance in rodents
Abstract
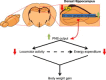
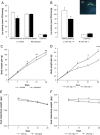
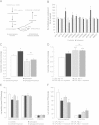
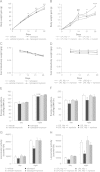
Brain lipid sensing is necessary to regulate energy balance. Lipoprotein lipase (LPL) may play a role in this process. We tested if hippocampal LPL regulated energy homeostasis in rodents by specifically attenuating LPL activity in the hippocampus of rats and mice, either by infusing a pharmacological inhibitor (tyloxapol), or using a genetic approach (adeno-associated virus expressing Cre-GFP injected into Lpl (lox/lox) mice). Decreased LPL activity by either method led to increased body weight gain due to decreased locomotor activity and energy expenditure, concomitant with increased parasympathetic tone (unchanged food intake). Decreased LPL activity in both models was associated with increased de novo ceramide synthesis and neurogenesis in the hippocampus, while intrahippocampal infusion of de novo ceramide synthesis inhibitor myriocin completely prevented body weight gain. We conclude that hippocampal lipid sensing might represent a core mechanism for energy homeostasis regulation through de novo ceramide synthesis.
Keywords: AAV, adeno-associated virus; ANS, autonomic nervous system; CERS, ceramide synthase; CNS, central nervous system; Ceramides; Energy expenditure; GFP, green fluorescent protein; LPL, lipoprotein lipase; Lipid sensing; Obesity; Parasympathetic nervous system; RQ, respiratory quotient; SMPD1, acid sphingomyelin phosphodiesterase 1; SPHK1, sphingosine kinase 1; SPT, serine palmitoyltransferase; TG, triglycerides.
Figures
References
-
- Luquet S., Magnan C. The central nervous system at the core of the regulation of energy homeostasis. Frontiers in Bioscience (Scholar Edition) 2009;1:448–465. - PubMed
-
- Sanchez-Lasheras C., Konner A.C., Bruning J.C. Integrative neurobiology of energy homeostasis-neurocircuits, signals and mediators. Frontiers in Neuroendocrinology. 2010;31(1):4–15. - PubMed
-
- Blouet C., Schwartz G.J. Hypothalamic nutrient sensing in the control of energy homeostasis. Behavioural Brain Research. 2010;209(1):1–12. - PubMed
-
- Cowley M.A. Hypothalamic melanocortin neurons integrate signals of energy state. European Journal of Pharmacology. 2003;480(1–3):3–11. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

