Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency
- PMID: 24634822
- PMCID: PMC3953682
- DOI: 10.1016/j.molmet.2013.11.010
Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency
Abstract
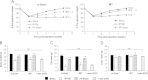
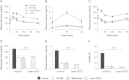
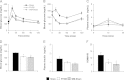
Glucagon-like peptide-1 (GLP-1) secretion is greatly enhanced after Roux-en-Y gastric bypass (RYGB). While intact GLP-1exerts its metabolic effects via the classical GLP-1 receptor (GLP-1R), proteolytic processing of circulating GLP-1 yields metabolites such as GLP-1(9-36)amide/GLP-1(28-36)amide, that exert similar effects independent of the classical GLP-1R. We investigated the hypothesis that GLP-1, acting via these metabolites or through its known receptor, is required for the beneficial effects of RYGB using two models of functional GLP-1 deficiency - α-gustducin-deficient (α-Gust (-/-)) mice, which exhibit attenuated nutrient-stimulated GLP-1 secretion, and GLP-1R-deficient mice. We show that the effect of RYGB to enhance glucose-stimulated GLP-1 secretion was greatly attenuated in α-Gust (-/-) mice. In both genetic models, RYGB reduced body weight and improved glucose homeostasis to levels observed in lean control mice. Therefore, GLP-1, acting through its classical GLP-1R or its bioactive metabolites, does not seem to be involved in the effects of RYGB on body weight and glucose homeostasis.
Keywords: GLP-1, glucagon-like peptide-1; GLP-1R, glucagon-like peptide-1 receptor; Glp1r−/−, glucagon-like peptide-1 receptor deficient mice; Gut hormones; HOMA-IR, Homeostasis Model Assessment-Insulin Resistance; Mouse model; PF-sham, pair-fed sham; RYGB, Roux-en-Y gastric bypass; Taste perception; WM-sham, weight-matched sham; WT, wild-type; Weight-loss surgery; α-Gust−/−, α-gustducin deficient mice.
Figures



References
-
- Mingrone G., Panunzi S., De Gaetano A., Guidone C., Iaconelli A., Leccesi L., Nanni G., Pomp A., Castagneto M., Ghirlanda G., Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. New England Journal of Medicine. 2012;366:1577–1585. - PubMed
-
- Nguyen N.T., Masoomi H., Magno C.P., Nguyen X.M., Laugenour K., Lane J. Trends in use of bariatric surgery, 2003–2008. Journal of the American College of Surgeons. 2011;213:261–266. - PubMed
-
- Buchwald H., Estok R., Fahrbach K., Banel D., Jensen M.D., Pories W.J., Bantle J.P., Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. American Journal of Medicine. 2009;122:248–256. - PubMed
-
- le Roux C.W., Welbourn R., Werling M., Osborne A., Kokkinos A., Laurenius A., Lonroth H., Fandriks L., Ghatei M.A., Bloom S.R., Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Annals of Surgery. 2007;246:780–785. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

