The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis
- PMID: 24637114
- PMCID: PMC3967045
- DOI: 10.1101/gad.237081.113
The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis
Abstract
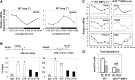
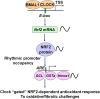
The disruption of the NRF2 (nuclear factor erythroid-derived 2-like 2)/glutathione-mediated antioxidant defense pathway is a critical step in the pathogenesis of several chronic pulmonary diseases and cancer. While the mechanism of NRF2 activation upon oxidative stress has been widely investigated, little is known about the endogenous signals that regulate the NRF2 pathway in lung physiology and pathology. Here we show that an E-box-mediated circadian rhythm of NRF2 protein is essential in regulating the rhythmic expression of antioxidant genes involved in glutathione redox homeostasis in the mouse lung. Using an in vivo bleomycin-induced lung fibrosis model, we reveal a clock "gated" pulmonary response to oxidative injury, with a more severe fibrotic effect when bleomycin was applied at a circadian nadir in NRF2 levels. Timed administration of sulforaphane, an NRF2 activator, significantly blocked this phenotype. Moreover, in the lungs of the arrhythmic Clock(Δ19) mice, the levels of NRF2 and the reduced glutathione are constitutively low, associated with increased protein oxidative damage and a spontaneous fibrotic-like pulmonary phenotype. Our findings reveal a pivotal role for the circadian control of the NRF2/glutathione pathway in combating oxidative/fibrotic lung damage, which might prompt new chronotherapeutic strategies for the treatment of human lung diseases, including idiopathic pulmonary fibrosis.
Keywords: NRF2; bleomycin; circadian clock; glutathione; pulmonary fibrosis.
Figures





References
-
- Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Römer S, Boutten A, et al. 2013. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal 18: 66–79 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
