Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets
- PMID: 24637292
- PMCID: PMC4005986
- DOI: 10.1016/j.ajpath.2014.01.035
Gastrointestinal pathology in juvenile and adult CFTR-knockout ferrets
Abstract
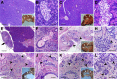
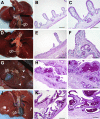
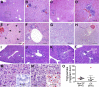
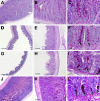
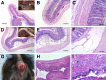
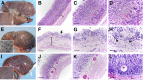
Cystic fibrosis (CF) is a multiorgan disease caused by loss of a functional cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel in many epithelia of the body. Here we report the pathology observed in the gastrointestinal organs of juvenile to adult CFTR-knockout ferrets. CF gastrointestinal manifestations included gastric ulceration, intestinal bacterial overgrowth with villous atrophy, and rectal prolapse. Metagenomic phylogenetic analysis of fecal microbiota by deep sequencing revealed considerable genotype-independent microbial diversity between animals, with the majority of taxa overlapping between CF and non-CF pairs. CF hepatic manifestations were variable, but included steatosis, necrosis, biliary hyperplasia, and biliary fibrosis. Gallbladder cystic mucosal hyperplasia was commonly found in 67% of CF animals. The majority of CF animals (85%) had pancreatic abnormalities, including extensive fibrosis, loss of exocrine pancreas, and islet disorganization. Interestingly, 2 of 13 CF animals retained predominantly normal pancreatic histology (84% to 94%) at time of death. Fecal elastase-1 levels from these CF animals were similar to non-CF controls, whereas all other CF animals evaluated were pancreatic insufficient (<2 μg elastase-1 per gram of feces). These findings suggest that genetic factors likely influence the extent of exocrine pancreas disease in CF ferrets and have implications for the etiology of pancreatic sufficiency in CF patients. In summary, these studies demonstrate that the CF ferret model develops gastrointestinal pathology similar to CF patients.
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Rowe S.M., Miller S., Sorscher E.J. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. - PubMed
-
- Robertson M.B., Choe K.A., Joseph P.M. Review of the abdominal manifestations of cystic fibrosis in the adult patient. Radiographics. 2006;26:679–690. - PubMed
-
- Oppenheimer E.H., Esterly J.R. Pathology of cystic fibrosis review of the literature and comparison with 146 autopsied cases. Perspect Pediatr Pathol. 1975;2:241–278. - PubMed
-
- Grubb B.R., Boucher R.C. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79:S193–S214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

