Androgen dynamics and serum PSA in patients treated with abiraterone acetate
- PMID: 24637537
- PMCID: PMC4020277
- DOI: 10.1038/pcan.2014.8
Androgen dynamics and serum PSA in patients treated with abiraterone acetate
Abstract
Background: We analyzed the potential of abiraterone acetate (henceforth abiraterone) to reduce androgen levels below lower limits of quantification (LLOQ) and explored the association with changes in PSA decline in metastatic castration-resistant prostate cancer (mCRPC) patients.
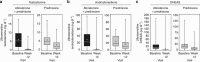
Methods: COU-AA-301 is a 2:1 randomized, double-blind, placebo-controlled study comparing abiraterone (1000 mg q.d.) plus low-dose prednisone (5 mg b.i.d.) with placebo plus prednisone in mCRPC patients post docetaxel. Serum testosterone, androstenedione and dehydroepiandrosterone sulfate from baseline to week 12 were measured by novel ultrasensitive two-dimensional liquid chromatography coupled to tandem mass spectrometry assays in a subset of subjects in each arm (abiraterone plus prednisone, n=80; prednisone, n=38). The association between PSA response (< or =50% baseline) and undetectable androgens (week 12 androgen level below LLOQ) was analyzed using logistic regression.
Results: A significantly greater reduction in serum androgens was observed with abiraterone plus prednisone versus prednisone (all P < or = 0.0003), reaching undetectable levels for testosterone (47.2% versus 0%, respectively). A positive association was observed between achieving undetectable serum androgens and PSA decline (testosterone: odds ratio=1.54; 95% confidence interval: 0.546-4.347). Reduction of androgens to undetectable levels did not occur in all patients achieving a PSA response, and a PSA response did not occur in all patients achieving undetectable androgen levels.
Conclusions: Abiraterone plus prednisone significantly reduced serum androgens, as measured by ultrasensitive assays and was generally associated with PSA response. However, androgen decline did not uniformly predict PSA decline suggesting ligand-independent or other mechanisms for mCRPC progression.
Figures


References
-
- Bonkhoff H, Berges R. From pathogenesis to prevention of castration resistant prostate cancer. Prostate. 2010;70:100–112. - PubMed
-
- Van Allen EM, Ryan CJ. Novel secondary hormonal therapy in advanced prostate cancer: an update. Curr Opin Urol. 2009;19:315–321. - PubMed
-
- Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–1264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

