Proteobacteria-specific IgA regulates maturation of the intestinal microbiota
- PMID: 24637807
- PMCID: PMC4049932
- DOI: 10.4161/gmic.26489
Proteobacteria-specific IgA regulates maturation of the intestinal microbiota
Abstract
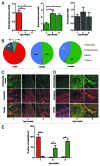
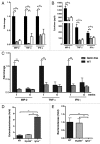
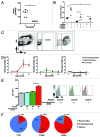
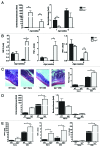
The intestinal microbiota changes dynamically from birth to adulthood. In this study we identified γ-Proteobacteria as a dominant phylum present in newborn mice that is suppressed in normal adult microbiota. The transition from a neonatal to a mature microbiota was in part regulated by induction of a γ-Proteobacteria-specific IgA response. Neocolonization experiments in germ-free mice further revealed a dominant Proteobacteria-specific IgA response triggered by the immature microbiota. Finally, a role for B cells in the regulation of microbiota maturation was confirmed in IgA-deficient mice. Mice lacking IgA had persistent intestinal colonization with γ-Proteobacteria that resulted in sustained intestinal inflammation and increased susceptibility to neonatal and adult models of intestinal injury. Collectively, these results identify an IgA-dependent mechanism responsible for the maturation of the intestinal microbiota.
Keywords: IgA; colitis; microbiota; necrotizing enterocolitis; proteobacteria.
Figures
References
-
- Carvalho FA, Koren O, Goodrich JK, Johansson ME, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, González A, et al. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12:139–52. doi: 10.1016/j.chom.2012.07.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
