Visualization of specific DNA sequences in living mouse embryonic stem cells with a programmable fluorescent CRISPR/Cas system
- PMID: 24637835
- PMCID: PMC4049922
- DOI: 10.4161/nucl.28488
Visualization of specific DNA sequences in living mouse embryonic stem cells with a programmable fluorescent CRISPR/Cas system
Abstract
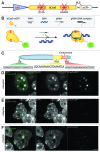
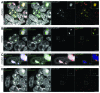
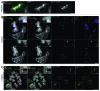
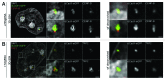
Labeling and tracing of specific sequences in living cells has been a major challenge in studying the spatiotemporal dynamics of native chromatin. Here we repurposed the prokaryotic CRISPR/Cas adaptive immunity system to specifically detect endogenous genomic loci in mouse embryonic stem cells. We constructed a catalytically inactive version of the Cas9 endonuclease, fused it with eGFP (dCas9-eGFP) and co-expressed small guide RNAs (gRNAs) to target pericentric, centric, and telomeric repeats, which are enriched in distinct nuclear structures. With major satellite specific gRNAs we obtained a characteristic chromocenter (CC) pattern, while gRNAs targeting minor satellites and telomeres highlighted smaller foci coinciding with centromere protein B (CENP-B) and telomeric repeat-binding factor 2 (TRF2), respectively. DNA sequence specific labeling by gRNA/dCas9-eGFP complexes was directly shown with 3D-fluorescent in situ hybridization (3D-FISH). Structured illumination microscopy (3D-SIM) of gRNA/dCas9-eGFP expressing cells revealed chromatin ultrastructures and demonstrated the potential of this approach for chromatin conformation studies by super resolution microscopy. This programmable dCas9 labeling system opens new perspectives to study functional nuclear architecture.
Keywords: 3D-SIM; 3D-fluorescent in situ hybridization (3D-FISH); CENP-B; CRISPR/Cas9; DNA labeling; TRF2; embryonic stem cells; major satellite repeats; minor satellite repeats; telomeres.
Figures
References
-
- Martin RM, Leonhardt H, Cardoso MC. DNA labeling in living cells. Cytometry Part A: the journal of the International Society for Analytical Cytology 2005; 67:45-52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
