Utility of gene expression profiling score variability to predict clinical events in heart transplant recipients
- PMID: 24637869
- PMCID: PMC3983476
- DOI: 10.1097/01.TP.0000443897.29951.cf
Utility of gene expression profiling score variability to predict clinical events in heart transplant recipients
Abstract
Background: Gene expression profiling test scores have primarily been used to identify heart transplant recipients who have a low probability of rejection at the time of surveillance testing. We hypothesized that the variability of gene expression profiling test scores within a patient may predict risk of future events of allograft dysfunction or death.
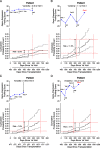
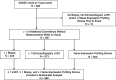
Method: Patients from the IMAGE study with rejection surveillance gene expression profiling tests performed at 1- to 6-month intervals were selected for this cohort study. Gene expression profiling score variability was defined as the standard deviation of an individual's cumulative test scores. Gene expression profiling ordinal score (range, 0-39), threshold score (binary value=1 if ordinal score ≥ 34), and score variability were studied in multivariate Cox regression models to predict future clinical events.
Results: Race, age at time of transplantation, and time posttransplantation were significantly associated with future events in the univariate analysis. In the multivariate analyses, gene expression profiling score variability, but not ordinal scores or scores over threshold, was independently associated with future clinical events. The regression coefficient P values were <0.001, 0.46, and 0.773, for gene expression profiling variability, ordinal, and threshold scores, respectively. The hazard ratio for a 1 unit increase in variability was 1.76 (95% CI, 1.4-2.3).
Discussion: The variability of a heart recipient's gene expression profiling test scores over time may provide prognostic utility. This information is independent of the probability of acute cellular rejection at the time of testing that is rendered from a single ordinal gene-expression profiling test score.
Trial registration: ClinicalTrials.gov NCT00351559.
Figures
References
-
- Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010; 29: 914. - PubMed
-
- Kirklin JK. Is biopsy-proven cellular rejection an important clinical consideration in heart transplantation? Curr Opin Cardiol 2005; 20: 127. - PubMed
-
- Hamour IM, Burke MM, Bell AD, et al. Limited utility of endomyocardial biopsy in the first year after heart transplantation. Transplantation 2008; 85: 969. - PubMed
-
- Deng MC, Eisen HJ, Mehra MR, et al. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant 2006; 6: 150. - PubMed
-
- Starling RC, Pham M, Valantine H, et al. Molecular testing in the management of cardiac transplant recipients: initial clinical experience. J Heart Lung Transplant 2007; 26: 204 - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

