Generation of neural progenitor cells by chemical cocktails and hypoxia
- PMID: 24638034
- PMCID: PMC4042166
- DOI: 10.1038/cr.2014.32
Generation of neural progenitor cells by chemical cocktails and hypoxia
Erratum in
-
Generation of neural progenitor cells by chemical cocktails and hypoxia.Cell Res. 2015 May;25(5):645-6. doi: 10.1038/cr.2015.55. Cell Res. 2015. PMID: 25941173 Free PMC article. No abstract available.
Abstract
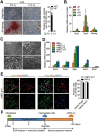
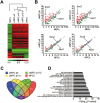
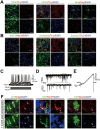
Neural progenitor cells (NPCs) can be induced from somatic cells by defined factors. Here we report that NPCs can be generated from mouse embryonic fibroblasts by a chemical cocktail, namely VCR (V, VPA, an inhibitor of HDACs; C, CHIR99021, an inhibitor of GSK-3 kinases and R, Repsox, an inhibitor of TGF-β pathways), under a physiological hypoxic condition. These chemical-induced NPCs (ciNPCs) resemble mouse brain-derived NPCs regarding their proliferative and self-renewing abilities, gene expression profiles, and multipotency for different neuroectodermal lineages in vitro and in vivo. Further experiments reveal that alternative cocktails with inhibitors of histone deacetylation, glycogen synthase kinase, and TGF-β pathways show similar efficacies for ciNPC induction. Moreover, ciNPCs can also be induced from mouse tail-tip fibroblasts and human urinary cells with the same chemical cocktail VCR. Thus our study demonstrates that lineage-specific conversion of somatic cells to NPCs could be achieved by chemical cocktails without introducing exogenous factors.
Figures



Comment in
-
A chemical approach to "rewire" neural progenitor cells.Cell Res. 2014 Jun;24(6):641-2. doi: 10.1038/cr.2014.51. Epub 2014 Apr 18. Cell Res. 2014. PMID: 24743789 Free PMC article.
References
-
- Gilbert S.Developmental Biology6th Edition: Sunderland: Sinauer Associates 2000.
-
- Gurdon JB, Elsdale TR, Fischberg M. Sexually mature individuals of Xenopus laevis from the transplantation of single somatic nuclei. Nature. 1958;182:64–65. - PubMed
-
- Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. - PubMed
-
- Miller RA, Ruddle FH. Pluripotent teratocarcinoma-thymus somatic cell hybrids. Cell. 1976;9:45–55. - PubMed
-
- Blau HM, Chiu CP, Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983;32:1171–1180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

