Loss of Dab2 expression in breast cancer cells impairs their ability to deplete TGF-β and induce Tregs development via TGF-β
- PMID: 24638085
- PMCID: PMC3956763
- DOI: 10.1371/journal.pone.0091709
Loss of Dab2 expression in breast cancer cells impairs their ability to deplete TGF-β and induce Tregs development via TGF-β
Abstract
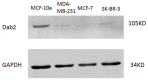
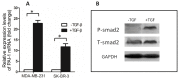
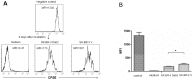
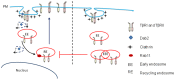
Dab2 is a multifunctional adapter protein which is frequently under-expressed in a variety of cancers. It is implicated in many critical functions, including several signaling pathways, cell arrangement, differentiation of stem cells, and receptor endocytosis. Transforming growth factor-β (TGF-β) is a secreted multifunctional protein that controls several developmental processes and pathogenesis of many diseases. It has been documented that Dab2 played an important role in TGF-β receptors endocytosis. Here, we present evidence that re-expression of Dab2 in SK-BR-3 cell partially restored its ability to deplete TGF-β in surrounding medium by normalizing the trafficking of TGF-β receptors. We also demonstrate that the difference in TGF-β depletions produced by Dab2 expression was sufficient to impact on the conversion of naive CD4+ T cells to regulatory T cells (Tregs), and thus inhibited the proliferation of T cells. This work revealed a critical result that breast cancer cell was deficient in Dab2 expression and related receptor endocytosis-mediated TGF-β depletion, which may contribute to the accumulation of TGF-β in tumor microenvironment and the induction of immune tolerance.
Conflict of interest statement
Figures





References
-
- Jemal A, Bray F (2011) Center MM, Ferlay J, Ward E, et al (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. - PubMed
-
- Sheng Z, He J, Tuppen JA, Sun W, Fazili Z, et al. (2000) Structure, sequence, and promoter analysis of human disabled-2 gene (DAB2). Genomics 70: 381–386. - PubMed
-
- Zhou J, Hsieh JT (2001) The inhibitory role of DOC-2/DAB2 in growth factor receptor-mediated signal cascade. DOC-2/DAB2-mediated inhibition of ERK phosphorylation via binding to Grb2. J Biol Chem 276: 27793–27798. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

