A phase II, multicentre trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal-related events
- PMID: 24638849
- PMCID: PMC3962742
- DOI: 10.1007/s10549-014-2906-x
A phase II, multicentre trial evaluating the efficacy of de-escalated bisphosphonate therapy in metastatic breast cancer patients at low-risk of skeletal-related events
Abstract
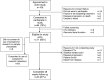
The optimal frequency of intravenous (IV) bisphosphonate administration is unclear. We thus performed a study evaluating the effects of switching from 3-4 to 12 weekly therapy in patients with biochemically defined low-risk bone metastases. Patients with serum C-telopeptide (CTx) levels ≤600 ng/L after ≥3 months of 3-4 weekly IV pamidronate were switched to 12 weekly therapy for 48 weeks. Primary endpoint was the proportion of patients maintaining CTx levels in the lower-risk range. All endpoints (serum CTx and bone-specific alkaline phosphatase (BSAP), skeletal-related events (SREs) and self-reported pain) were measured at baseline, 6, 12, 24, 36 and 48 weeks. Treatment failure was defined as biochemical failure (CTx > 600 ng/L) or a SRE. Exploratory biomarkers including; serum TGF-β, activin-A, bone sialoprotein (BSP), procollagen type 1 N-terminal propeptide and urinary N-telopeptide (NTx) were assessed at baseline as predictors for failure to complete treatment. Seventy-one patients accrued and 43 (61 %) completed 48 weeks of de-escalated therapy. Reasons for failure to complete treatment included; biochemical failure (CTx > 600 ng/L) (n = 10, 14.1 %), on-study SRE (n = 9, 12.7 %), disease progression (n = 7, 9.9 % including death from disease [n = 1, 1.4 %]) or patient choice (n = 2, 2.8 %). Elevated baseline levels of CTx, BSAP, NTx and BSP were associated with treatment failure. The majority of patients in this biochemically defined low-risk population could switch from 3-4 weekly to 12 weekly bisphosphonate therapy with no effect on CTx levels or SREs during the 48 week study. Larger trials are required to assess the roles of biomarkers as predictors of adequacy of de-escalated therapy.
References
-
- Verma S, Kerr-Cresswell D, Dranitsaris G, Charbonneau F, Trudeau M, Yogendran G, Cesta AM, Clemons M. Bisphosphonate use for the management of breast cancer patients with bone metastases: a survey of Canadian Medical Oncologists. Support Care Cancer. 2004;12(12):852–858. doi: 10.1007/s00520-004-0671-9. - DOI - PubMed
-
- Clemons M, Enright K, Cesta A, Charbonneau F, Chow E, Warr D, Kee-Cresswell D, Chang J, Yogendran G, Trudeau M, De Angelis C, Cottrell W, Dranitsaris G. Do physicians follow systemic treatment and funding policy guidelines? Can J Clin Pharmacol. 2004;11(1):e168–e178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous