Behavioral flexibility is increased by optogenetic inhibition of neurons in the nucleus accumbens shell during specific time segments
- PMID: 24639489
- PMCID: PMC3966536
- DOI: 10.1101/lm.034199.113
Behavioral flexibility is increased by optogenetic inhibition of neurons in the nucleus accumbens shell during specific time segments
Abstract
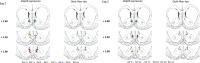
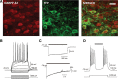
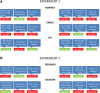
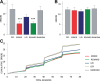
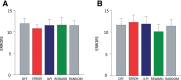
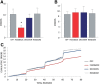
Behavioral flexibility is vital for survival in an environment of changing contingencies. The nucleus accumbens may play an important role in behavioral flexibility, representing learned stimulus-reward associations in neural activity during response selection and learning from results. To investigate the role of nucleus accumbens neural activity in behavioral flexibility, we used light-activated halorhodopsin to inhibit nucleus accumbens shell neurons during specific time segments of a bar-pressing task requiring a win-stay/lose-shift strategy. We found that optogenetic inhibition during action selection in the time segment preceding a lever press had no effect on performance. However, inhibition occurring in the time segment during feedback of results--whether rewards or nonrewards--reduced the errors that occurred after a change in contingency. Our results demonstrate critical time segments during which nucleus accumbens shell neurons integrate feedback into subsequent responses. Inhibiting nucleus accumbens shell neurons in these time segments, during reinforced performance or after a change in contingencies, increases lose-shift behavior. We propose that the activity of nucleus shell accumbens shell neurons in these time segments plays a key role in integrating knowledge of results into subsequent behavior, as well as in modulating lose-shift behavior when contingencies change.
Figures

References
-
- Aggarwal M, Hyland BI, Wickens JR 2012. Neural control of dopamine neurotransmission: implications for reinforcement learning. Eur J Neurosci 35: 1115–1123 - PubMed
-
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K 2007. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng 4: S143–S156 - PubMed
-
- Barto AG, Sutton RS 1982. Simulation of anticipatory responses in classical conditioning by a neuron-like adaptive element. Behav Brain Res 4: 221–235 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
