Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing
- PMID: 24639501
- PMCID: PMC3977267
- DOI: 10.1073/pnas.1403244111
Cartography of neurexin alternative splicing mapped by single-molecule long-read mRNA sequencing
Abstract
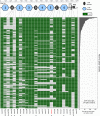
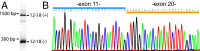
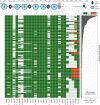
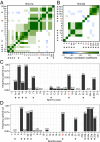
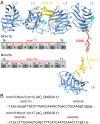
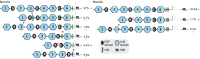
Neurexins are evolutionarily conserved presynaptic cell-adhesion molecules that are essential for normal synapse formation and synaptic transmission. Indirect evidence has indicated that extensive alternative splicing of neurexin mRNAs may produce hundreds if not thousands of neurexin isoforms, but no direct evidence for such diversity has been available. Here we use unbiased long-read sequencing of full-length neurexin (Nrxn)1α, Nrxn1β, Nrxn2β, Nrxn3α, and Nrxn3β mRNAs to systematically assess how many sites of alternative splicing are used in neurexins with a significant frequency, and whether alternative splicing events at these sites are independent of each other. In sequencing more than 25,000 full-length mRNAs, we identified a novel, abundantly used alternatively spliced exon of Nrxn1α and Nrxn3α (referred to as alternatively spliced sequence 6) that encodes a 9-residue insertion in the flexible hinge region between the fifth LNS (laminin-α, neurexin, sex hormone-binding globulin) domain and the third EGF-like sequence. In addition, we observed several larger-scale events of alternative splicing that deleted multiple domains and were much less frequent than the canonical six sites of alternative splicing in neurexins. All of the six canonical events of alternative splicing appear to be independent of each other, suggesting that neurexins may exhibit an even larger isoform diversity than previously envisioned and comprise thousands of variants. Our data are consistent with the notion that α-neurexins represent extracellular protein-interaction scaffolds in which different LNS and EGF domains mediate distinct interactions that affect diverse functions and are independently regulated by independent events of alternative splicing.
Keywords: LRRTM; autism; cerebellin; neuroligin; schizophrenia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10(6):483–490. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

