Synaptic vesicle tethering and the CaV2.2 distal C-terminal
- PMID: 24639630
- PMCID: PMC3945931
- DOI: 10.3389/fncel.2014.00071
Synaptic vesicle tethering and the CaV2.2 distal C-terminal
Abstract
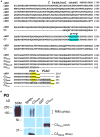
Evidence that synaptic vesicles (SVs) can be gated by a single voltage sensitive calcium channel (CaV2.2) predict a molecular linking mechanism or "tether" (Stanley, 1993). Recent studies have proposed that the SV binds to the distal C-terminal on the CaV2.2 calcium channel (Kaeser et al., 2011; Wong et al., 2013) while genetic analysis proposed a double tether mechanism via RIM: directly to the C terminus PDZ ligand domain or indirectly via a more proximal proline rich site (Kaeser et al., 2011). Using a novel in vitro SV pull down binding assay, we reported that SVs bind to a fusion protein comprising the C-terminal distal third (C3, aa 2137-2357; Wong et al., 2013). Here we limit the binding site further to the last 58 aa, beyond the proline rich site, by the absence of SV capture by a truncated C3 fusion protein (aa 2137-2299). To test PDZ-dependent binding we generated two C terminus-mutant C3 fusion proteins and a mimetic blocking peptide (H-WC, aa 2349-2357) and validated these by elimination of MINT-1 or RIM binding. Persistence of SV capture with all three fusion proteins or with the full length C3 protein but in the presence of blocking peptide, demonstrated that SVs can bind to the distal C-terminal via a PDZ-independent mechanism. These results were supported in situ by normal SV turnover in H-WC-loaded synaptosomes, as assayed by a novel peptide cryoloading method. Thus, SVs tether to the CaV2.2 C-terminal within a 49 aa region immediately prior to the terminus PDZ ligand domain. Long tethers that could reflect extended C termini were imaged by electron microscopy of synaptosome ghosts. To fully account for SV tethering we propose a model where SVs are initially captured, or "grabbed," from the cytoplasm by a binding site on the distal region of the channel C-terminal and are then retracted to be "locked" close to the channel by a second attachment mechanism in preparation for single channel domain gating.
Keywords: PDZ; RIM binding protein; SV-PD; calcium channel; cryoloading; presynaptic; synaptic vesicle; tether.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

