Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia
- PMID: 24641185
- PMCID: PMC3952799
- DOI: 10.1111/bph.12489
Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia
Abstract
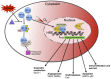
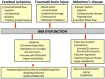
The blood-brain barrier (BBB) is a complex vascular structure consisting of microvascular endothelial cells that line the vessel wall, astrocyte end-feet, pericytes, as well as the basal lamina. BBB cells act in concert to maintain the characteristic impermeable and low paracellular flux of the brain vascular network, thus ensuring a homeostatic neuronal environment. Alterations in BBB stability that occur during injury have dire consequences on disease progression and it is clear that BBB cell-specific responses, positive or negative, must make a significant contribution to injury outcome. Reduced oxygenation, or hypoxia, is a characteristic of many brain diseases that significantly increases barrier permeability. Recent data suggest that hypoxia-inducible factor (HIF-1), the master regulator of the hypoxic response, probably mediates many hypoxic effects either directly or indirectly via its target genes. This review discusses current knowledge of physiological cell-specific regulation of barrier function, their responses to hypoxia as well as consequences of hypoxic- and HIF-1-mediated mechanisms on barrier integrity during select brain diseases. In the final sections, the potential of current advances in targeting HIF-1 as a therapeutic strategy will be overviewed.
Keywords: BBB cell-specific response; HIF-1; astrocytes; endothelial cells; hypoxia; neurodegenerative diseases; pericytes.
© 2013 The British Pharmacological Society.
Figures

References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. - PubMed
-
- Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther. 1999a;289:668–675. - PubMed
-
- Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999b;842:277–286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

