Transcriptional regulation induced by cAMP elevation in mouse Schwann cells
- PMID: 24641305
- PMCID: PMC4834722
- DOI: 10.1042/AN20130031
Transcriptional regulation induced by cAMP elevation in mouse Schwann cells
Abstract
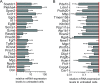
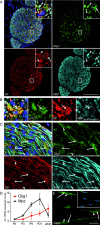
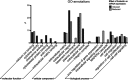
In peripheral nerves, Schwann cell development is regulated by a variety of signals. Some of the aspects of Schwann cell differentiation can be reproduced in vitro in response to forskolin, an adenylyl cyclase activator elevating intracellular cAMP levels. Herein, the effect of forskolin treatment was investigated by a comprehensive genome-wide expression study on primary mouse Schwann cell cultures. Additional to myelin-related genes, many so far unconsidered genes were ascertained to be modulated by forskolin. One of the strongest differentially regulated gene transcripts was the transcription factor Olig1 (oligodendrocyte transcription factor 1), whose mRNA expression levels were reduced in treated Schwann cells. Olig1 protein was localized in myelinating and nonmyelinating Schwann cells within the sciatic nerve as well as in primary Schwann cells, proposing it as a novel transcription factor of the Schwann cell lineage. Data analysis further revealed that a number of differentially expressed genes in forskolin-treated Schwann cells were associated with the ECM (extracellular matrix), underlining its importance during Schwann cell differentiation in vitro. Comparison of samples derived from postnatal sciatic nerves and from both treated and untreated Schwann cell cultures showed considerable differences in gene expression between in vivo and in vitro, allowing us to separate Schwann cell autonomous from tissue-related changes. The whole data set of the cell culture microarray study is provided to offer an interactive search tool for genes of interest.
Figures


Similar articles
-
MAL overexpression leads to disturbed expression of genes that influence cytoskeletal organization and differentiation of Schwann cells.ASN Neuro. 2014 Sep 10;6(5):1759091414548916. doi: 10.1177/1759091414548916. Print 2014. ASN Neuro. 2014. PMID: 25290060 Free PMC article.
-
The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition.J Cell Biol. 1991 Feb;112(3):457-67. doi: 10.1083/jcb.112.3.457. J Cell Biol. 1991. PMID: 1704008 Free PMC article.
-
Protein kinase A-induced phosphorylation of the p65 subunit of nuclear factor-kappaB promotes Schwann cell differentiation into a myelinating phenotype.J Neurosci. 2008 Apr 2;28(14):3738-46. doi: 10.1523/JNEUROSCI.4439-07.2008. J Neurosci. 2008. PMID: 18385332 Free PMC article.
-
Tissue culture studies of Schwann cell proliferation and differentiation.Dev Neurosci. 1985;7(5-6):364-73. doi: 10.1159/000112303. Dev Neurosci. 1985. PMID: 2424706 Review.
-
Regulation of myelin-specific gene expression. Relevance to CMT1.Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review.
Cited by
-
Ethanol Extract of Aurantiochytrium mangrovei 18W-13a Strain Possesses Anti-inflammatory Effects on Murine Macrophage RAW264 Cells.Front Physiol. 2018 Sep 26;9:1205. doi: 10.3389/fphys.2018.01205. eCollection 2018. Front Physiol. 2018. PMID: 30319432 Free PMC article.
-
Meta-Analysis Reveals Transcription Factor Upregulation in Cells of Injured Mouse Sciatic Nerve.Front Cell Neurosci. 2021 Oct 21;15:688243. doi: 10.3389/fncel.2021.688243. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34744629 Free PMC article.
-
Calreticulin Promotes Proliferation and Migration But Inhibits Apoptosis in Schwann Cells.Med Sci Monit. 2016 Nov 23;22:4516-4522. doi: 10.12659/msm.900956. Med Sci Monit. 2016. PMID: 27876711 Free PMC article.
-
MAL overexpression leads to disturbed expression of genes that influence cytoskeletal organization and differentiation of Schwann cells.ASN Neuro. 2014 Sep 10;6(5):1759091414548916. doi: 10.1177/1759091414548916. Print 2014. ASN Neuro. 2014. PMID: 25290060 Free PMC article.
-
Direct Reprogramming of Spiral Ganglion Non-neuronal Cells into Neurons: Toward Ameliorating Sensorineural Hearing Loss by Gene Therapy.Front Cell Dev Biol. 2018 Feb 14;6:16. doi: 10.3389/fcell.2018.00016. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 29492404 Free PMC article.
References
-
- Afshari FT, Kwok JC, White L, Fawcett JW. Schwann cell migration is integrin-dependent and inhibited by astrocyte-produced aggrecan. Glia. 2010;58:857–869. - PubMed
-
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science. 2004;306:2111–2115. - PubMed
-
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, Wicher GK, Mitter R, Greensmith L, Behrens A, Raivich G, Mirsky R, Jessen KR. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. - PMC - PubMed
-
- Barradas M, Gonos ES, Zebedee Z, Kolettas E, Petropoulou C, Delgado MD, Leon J, Hara E, Serrano M. Identification of a candidate tumor-suppressor gene specifically activated during Ras-induced senescence. Exp Cell Res. 2002;273:127–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

