Suppression of cell membrane permeability by suramin: involvement of its inhibitory actions on connexin 43 hemichannels
- PMID: 24641330
- PMCID: PMC4105932
- DOI: 10.1111/bph.12693
Suppression of cell membrane permeability by suramin: involvement of its inhibitory actions on connexin 43 hemichannels
Abstract
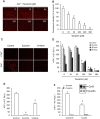
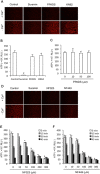
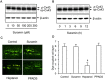
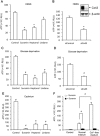
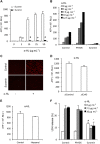
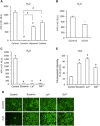
Background and purpose: Suramin is a clinically prescribed drug for treatment of human African trypanosomiasis, cancer and infection. It is also a well-known pharmacological antagonist of P2 purinoceptors. Despite its clinical use and use in research, the biological actions of this molecule are still incompletely understood. Here, we investigated the effects of suramin on membrane channels, as exemplified by its actions on non-junctional connexin43 (Cx43) hemichannels, pore-forming α-haemolysin and channels involved in ATP release under hypotonic conditions.
Experimental approach: Hemichannels were activated by removing extracellular Ca(2+) . The influences of suramin on hemichannel activities were evaluated by its effects on influx of fluorescent dyes and efflux of ATP. The membrane permeability and integrity were assessed through cellular retention of preloaded calcein and LDH release.
Key results: Suramin blocked Cx43 hemichannel permeability induced by removal of extracellular Ca(2+) without much effect on Cx43 expression and gap junctional intercellular communication. This action of suramin was mimicked by its analogue NF023 and NF449 but not by another P2 purinoceptor antagonist PPADS. Besides hemichannels, suramin also significantly blocked intracellular and extracellular exchanges of small molecules caused by α-haemolysin from Staphylococcus aureus and by exposure of cells to hypotonic solution. Furthermore, it prevented α-haemolysin- and hypotonic stress-elicited cell injury.
Conclusion and implications: Suramin blocked membrane channels and protected cells against toxin- and hypotonic stress-elicited injury. Our finding provides novel mechanistic insights into the pharmacological actions of suramin. Suramin might be therapeutically exploited to protect membrane integrity under certain pathological situations.
Keywords: channel permeability; connexin 43; hemichannels; hypotonic stress; suramin; α-haemolysin.
© 2014 The British Pharmacological Society.
Figures
Similar articles
-
At the cross-point of connexins, calcium, and ATP: blocking hemichannels inhibits vasoconstriction of rat small mesenteric arteries.Cardiovasc Res. 2017 Feb;113(2):195-206. doi: 10.1093/cvr/cvw215. Epub 2016 Sep 27. Cardiovasc Res. 2017. PMID: 27677282
-
Blocking Connexin-43 mediated hemichannel activity protects against early tubular injury in experimental chronic kidney disease.Cell Commun Signal. 2020 May 25;18(1):79. doi: 10.1186/s12964-020-00558-1. Cell Commun Signal. 2020. PMID: 32450899 Free PMC article.
-
Emerging issues of connexin channels: biophysics fills the gap.Q Rev Biophys. 2001 Aug;34(3):325-472. doi: 10.1017/s0033583501003705. Q Rev Biophys. 2001. PMID: 11838236 Review.
-
Purinergic control of AMPK activation by ATP released through connexin 43 hemichannels - pivotal roles in hemichannel-mediated cell injury.J Cell Sci. 2014 Apr 1;127(Pt 7):1487-99. doi: 10.1242/jcs.139089. Epub 2014 Feb 4. J Cell Sci. 2014. PMID: 24496445
-
Connexin targeting peptides as inhibitors of voltage- and intracellular Ca2+-triggered Cx43 hemichannel opening.Neuropharmacology. 2013 Dec;75:506-16. doi: 10.1016/j.neuropharm.2013.08.021. Epub 2013 Sep 2. Neuropharmacology. 2013. PMID: 24007825 Review.
Cited by
-
100 Years of Suramin.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01168-19. doi: 10.1128/AAC.01168-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31844000 Free PMC article. Review.
-
Connexin43 hemichannels contributes to the disassembly of cell junctions through modulation of intracellular oxidative status.Redox Biol. 2016 Oct;9:198-209. doi: 10.1016/j.redox.2016.08.008. Epub 2016 Aug 21. Redox Biol. 2016. PMID: 27567473 Free PMC article.
-
Connexin Hemichannels Contribute to the Activation of cAMP Signaling Pathway and Renin Production.Int J Mol Sci. 2020 Jun 23;21(12):4462. doi: 10.3390/ijms21124462. Int J Mol Sci. 2020. PMID: 32585970 Free PMC article.
-
Circadian coordination of ATP release in the urothelium via connexin43 hemichannels.Sci Rep. 2018 Jan 31;8(1):1996. doi: 10.1038/s41598-018-20379-0. Sci Rep. 2018. PMID: 29386573 Free PMC article.
-
Ameliorative Effect of Ginsenoside Rc on 5-Fluorouracil-Induced Chemotherapeutic Intestinal Mucositis via the PI3K-AKT/NF-κB Signaling Pathway: In Vivo and In Vitro Evaluations.Int J Mol Sci. 2024 Dec 5;25(23):13085. doi: 10.3390/ijms252313085. Int J Mol Sci. 2024. PMID: 39684797 Free PMC article.
References
-
- Bachmann A, Russ U, Quast U. Potent inhibition of the CFTR chloride channel by suramin. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:473–476. - PubMed
-
- Baraldi PG, del Carmen Nuñez M, Morelli A, Falzoni S, Di Virgilio F, Romagnoli R. Synthesis and biological activity of N-arylpiperazine-modified analogues of KN-62, a potent antagonist of the purinergic P2X7 receptor. J Med Chem. 2003;46:1318–1329. - PubMed
-
- Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

