Histamine mediates behavioural and metabolic effects of 3-iodothyroacetic acid, an endogenous end product of thyroid hormone metabolism
- PMID: 24641572
- PMCID: PMC4105934
- DOI: 10.1111/bph.12697
Histamine mediates behavioural and metabolic effects of 3-iodothyroacetic acid, an endogenous end product of thyroid hormone metabolism
Abstract
Background and purpose: 3-Iodothyroacetic acid (TA1) is an end product of thyroid hormone metabolism. So far, it is not known if TA1 is present in mouse brain and if it has any pharmacological effects.
Experimental approach: TA1 levels in mouse brain were measured by HPLC coupled to mass spectrometry. After i.c.v. administration of exogenous TA1 (0.4, 1.32 and 4 μg·kg(-1) ) to mice, memory acquisition-retention (passive avoidance paradigm with a light-dark box), pain threshold to thermal stimulus (51.5°C; hot plate test) and plasma glucose (glucorefractometer) were evaluated. Similar assays were performed in mice pretreated with s.c. injections of the histamine H1 receptor antagonist pyrilamine (10 mg·kg(-1) ) or the H2 receptor antagonist zolantidine (5 mg·kg(-1) ). TA1 (1.32 and 4 μg·kg(-1) ) was also given i.c.v. to mice lacking histidine decarboxylase (HDC(-/-) ) and the corresponding WT strain.
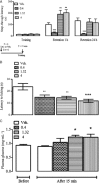
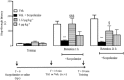
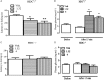
Key results: TA1 was found in the brain of CD1 but not of HDC mice. Exogenous TA1 induced amnesia (at 0.4 μg·kg(-1) ), stimulation of learning (1.32 and 4 μg·kg(-1) ), hyperalgesia (0.4, 1.32 and 4 μg·kg(-1) ) and hyperglycaemia (1.32 and 4 μg·kg(-1) ). All these effects were modulated by pyrilamine and zolantidine. In HDC(-/-) mice, TA1 (1.32 and 4 μg·kg(-1) ) did not increase plasma glucose or induce hyperalgesia.
Conclusions and implications: Behavioural and metabolic effects of TA1 disclosed interactions between the thyroid and histaminergic systems.
Keywords: 3-iodoacetic acid; anti-histaminergic drugs; histamine; learning; pain; thyromimetics.
© 2014 The British Pharmacological Society.
Figures

References
-
- Agretti P, De Marco G, Russo L, Saba A, Raffaelli A, Marchini M, et al. 3-Iodothyronamine metabolism and functional effects in FRTL5 thyroid cells. J Mol Endocrinol. 2011;47:23–32. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

