Accelerated microglial pathology is associated with Aβ plaques in mouse models of Alzheimer's disease
- PMID: 24641683
- PMCID: PMC4326940
- DOI: 10.1111/acel.12210
Accelerated microglial pathology is associated with Aβ plaques in mouse models of Alzheimer's disease
Abstract
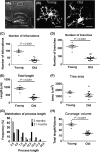
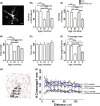
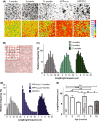
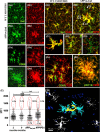
Microglia integrate within the neural tissue with a distinct ramified morphology through which they scan the surrounding neuronal network. Here, we used a digital tool for the quantitative morphometric characterization of fine cortical microglial structures in mice, and the changes they undergo with aging and in Alzheimer's-like disease. We show that, compared with microglia in young mice, microglia in old mice are less ramified and possess fewer branches and fine processes along with a slightly increased proinflammatory cytokine expression. A similar microglial pathology appeared 6-12 months earlier in mouse models of Alzheimer's disease (AD), along with a significant increase in brain parenchyma lacking coverage by microglial processes. We further demonstrate that microglia near amyloid plaques acquire unique activated phenotypes with impaired process complexity. We thus show that along with a chronic proinflammatory reaction in the brain, aging causes a significant reduction in the capacity of microglia to scan their environment. This type of pathology is markedly accelerated in mouse models of AD, resulting in a severe microglial process deficiency, and possibly contributing to enhanced cognitive decline.
Keywords: Alzheimer's disease; aging; amyloid beta-peptide; inflammation; microglia; morphology.
© 2014 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

References
-
- Abutbul S, Shapiro J, Szaingurten-Solodkin I, Levy N, Carmy Y, Baron R, Jung S, Monsonego A. TGF-beta signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia. 2012;60:1160–1171. - PubMed
-
- Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 2012;15:349–357. - PubMed
-
- Braun N, Sevigny J, Robson SC, Enjyoji K, Guckelberger O, Hammer K, Di Virgilio F, Zimmermann H. Assignment of ecto-nucleoside triphosphate diphosphohydrolase-1/cd39 expression to microglia and vasculature of the brain. Eur. J Neurosci. 2000;12:4357–4366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

