Elucidation of intrinsic biosynthesis yields using 13C-based metabolism analysis
- PMID: 24642094
- PMCID: PMC3994946
- DOI: 10.1186/1475-2859-13-42
Elucidation of intrinsic biosynthesis yields using 13C-based metabolism analysis
Abstract
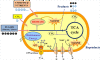
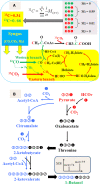
This paper discusses the use of 13C-based metabolism analysis for the assessment of intrinsic product yields - the actual carbon contribution from a single carbon substrate to the final product via a specific biosynthesis route - in the following four cases. First, undefined nutrients (such as yeast extract) in fermentation may contribute significantly to product synthesis, which can be quantified through an isotopic dilution method. Second, product and biomass synthesis may be dependent on the co-metabolism of multiple-carbon sources. 13C labeling experiments can track the fate of each carbon substrate in the cell metabolism and identify which substrate plays a main role in product synthesis. Third, 13C labeling can validate and quantify the contribution of the engineered pathway (versus the native pathway) to the product synthesis. Fourth, the loss of catabolic energy due to cell maintenance (energy used for functions other than production of new cell components) and low P/O ratio (Phosphate/Oxygen Ratio) significantly reduces product yields. Therefore, 13C-metabolic flux analysis is needed to assess the influence of suboptimal energy metabolism on microbial productivity, and determine how ATP/NAD(P)H are partitioned among various cellular functions. Since product yield is a major determining factor in the commercialization of a microbial cell factory, we foresee that 13C-isotopic labeling experiments, even without performing extensive flux calculations, can play a valuable role in the development and verification of microbial cell factories.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

