Effects of selective and non-selective glucocorticoid receptor II antagonists on rapid-onset diabetes in young rats
- PMID: 24642683
- PMCID: PMC3958344
- DOI: 10.1371/journal.pone.0091248
Effects of selective and non-selective glucocorticoid receptor II antagonists on rapid-onset diabetes in young rats
Abstract
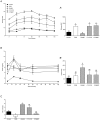
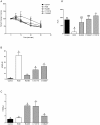
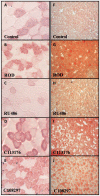
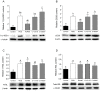
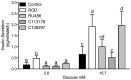
The blockade of glucocorticoid (GC) action through antagonism of the glucocorticoid receptor II (GRII) has been used to minimize the undesirable effects of chronically elevated GC levels. Mifepristone (RU486) is known to competitively block GRII action, but not exclusively, as it antagonizes the progesterone receptor. A number of new selective GRII antagonists have been developed, but limited testing has been completed in animal models of overt type 2 diabetes mellitus. Therefore, two selective GRII antagonists (C113176 and C108297) were tested to determine their effects in our model of GC-induced rapid-onset diabetes (ROD). Male Sprague-Dawley rats (∼ six weeks of age) were placed on a high-fat diet (60%), surgically implanted with pellets containing corticosterone (CORT) or wax (control) and divided into five treatment groups. Each group was treated with either a GRII antagonist or vehicle for 14 days after surgery: CORT pellets (400 mg/rat) + antagonists (80 mg/kg/day); CORT pellets + drug vehicle; and wax pellets (control) + drug vehicle. After 10 days of CORT treatment, body mass gain was increased with RU486 (by ∼20% from baseline) and maintained with C113176 administration, whereas rats given C108297 had similar body mass loss (∼15%) to ROD animals. Fasting glycemia was elevated in the ROD animals (>20 mM), normalized completely in animals treated with RU486 (6.2±0.1 mM, p<0.05) and improved in animals treated with C108297 and C113176 (14.0±1.6 and 8.8±1.6 mM, p<0.05 respectively). Glucose intolerance was normalized with RU486 treatment, whereas acute insulin response was improved with RU486 and C113176 treatment. Also, peripheral insulin resistance was attenuated with C113176 treatment along with improved levels of β-cell function while C108297 antagonism only provided modest improvements. In summary, C113176 is an effective agent that minimized some GC-induced detrimental metabolic effects and may provide an alternative to the effective, but non-selective, GRII antagonist RU486.
Conflict of interest statement
Figures

References
-
- Funder JW (1997) Glucocorticoid and mineralocorticoid receptors: Biology and clinical relevance. Annu Rev Med 48: 231–240. - PubMed
-
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998) Brain corticosteroid receptor balance in health and disease. Endocr Rev 19: 269–301. - PubMed
-
- Zhou J, Cidlowski JA (2005) The human glucocorticoid receptor: One gene, multiple proteins and diverse responses. Steroids 70: 407–417. - PubMed
-
- Rhen T, Cidlowski JA (2005) Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med 353: 1711–1723. - PubMed
-
- Andrews RC, Walker BR (1999) Glucocorticoids and insulin resistance: Old hormones, new targets. Clin Sci (Lond) 96: 513–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

