Increased calcification in osteoprotegerin-deficient smooth muscle cells: Dependence on receptor activator of NF-κB ligand and interleukin 6
- PMID: 24642764
- PMCID: PMC4057981
- DOI: 10.1159/000358920
Increased calcification in osteoprotegerin-deficient smooth muscle cells: Dependence on receptor activator of NF-κB ligand and interleukin 6
Abstract
Objective: Vascular calcification is highly correlated with cardiovascular disease morbidity and mortality. Osteoprotegerin (OPG) is a secreted decoy receptor for receptor activator of NF-κB ligand (RANKL). Inactivation of OPG in apolipoprotein E-deficient (ApoE-/-) mice increases lesion size and calcification. The mechanism(s) by which OPG is atheroprotective and anticalcific have not been entirely determined. We investigated whether OPG-deficient vascular smooth muscle cells (VSMCs) are more susceptible to mineralization and whether RANKL mediates this process.
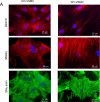
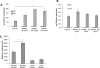
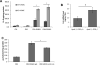
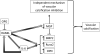
Results: Lesion-free aortas from 12-week-old ApoE-/-OPG-/- mice had spotty calcification, an appearance of osteochondrogenic factors and a decrease of smooth muscle markers when compared to ApoE-/-OPG+/+ aortas. In osteogenic conditions, VSMCs isolated from ApoE-/-OPG-/- (KO-VSMC) mice deposited more calcium than VSMCs isolated from ApoE-/-OPG+/+ (WT-VSMC) mice. Gene expression and biochemical analysis indicated accelerated osteochondrogenic differentiation. Ablation of RANKL signaling in KO-VSMCs rescued the accelerated calcification. While WT-VSMCs did not respond to RANKL treatment, KO-VSMCs responded with enhanced calcification and the upregulation of osteochondrogenic genes. RANKL strongly induced interleukin 6 (IL-6), which partially mediated RANKL-dependent calcification and gene expression in KO-VSMCs.
Conclusions: OPG inhibits vascular calcification by regulating the procalcific effects of RANKL on VSMCs and is thus a possible target for therapeutic intervention.
Figures
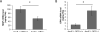
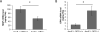






References
-
- Moe SM, Reslerova M, Ketteler M, O'neill K, Duan D, Koczman J, Westenfeld R, Jahnen-Dechent W, Chen NX. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (ckd). Kidney Int. 2005;67:2295–2304. - PubMed
-
- Abedin M, Tintut Y, Demer LL. Vascular calcification: Mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–1170. - PubMed
-
- Rattazzi M, Bennett BJ, Bea F, Kirk EA, Ricks JL, Speer M, Schwartz SM, Giachelli CM, Rosenfeld ME. Calcification of advanced atherosclerotic lesions in the innominate arteries of apoe-deficient mice: Potential role of chondrocyte-like cells. Arterioscler Thromb Vasc Biol. 2005;25:1420–1425. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

