N-glycosylation of effector proteins by an α-1,3-mannosyltransferase is required for the rice blast fungus to evade host innate immunity
- PMID: 24642938
- PMCID: PMC4001389
- DOI: 10.1105/tpc.114.123588
N-glycosylation of effector proteins by an α-1,3-mannosyltransferase is required for the rice blast fungus to evade host innate immunity
Abstract
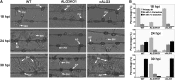
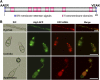
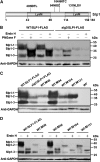
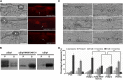
Plant pathogenic fungi deploy secreted effectors to suppress plant immunity responses. These effectors operate either in the apoplast or within host cells, so they are putatively glycosylated, but the posttranslational regulation of their activities has not been explored. In this study, the ASPARAGINE-LINKED GLYCOSYLATION3 (ALG3)-mediated N-glycosylation of the effector, Secreted LysM Protein1 (Slp1), was found to be essential for its activity in the rice blast fungus Magnaporthe oryzae. ALG3 encodes an α-1,3-mannosyltransferase for protein N-glycosylation. Deletion of ALG3 resulted in the arrest of secondary infection hyphae and a significant reduction in virulence. We observed that Δalg3 mutants induced massive production of reactive oxygen species in host cells, in a similar manner to Δslp1 mutants, which is a key factor responsible for arresting infection hyphae of the mutants. Slp1 sequesters chitin oligosaccharides to avoid their recognition by the rice (Oryza sativa) chitin elicitor binding protein CEBiP and the induction of innate immune responses, including reactive oxygen species production. We demonstrate that Slp1 has three N-glycosylation sites and that simultaneous Alg3-mediated N-glycosylation of each site is required to maintain protein stability and the chitin binding activity of Slp1, which are essential for its effector function. These results indicate that Alg3-mediated N-glycosylation of Slp1 is required to evade host innate immunity.
Figures




References
-
- Adam T., Bouhidel K., Der C., Robert F., Najid A., Simon-Plas F., Leborgne-Castel N. (2012). Constitutive expression of clathrin hub hinders elicitor-induced clathrin-mediated endocytosis and defense gene expression in plant cells. FEBS Lett. 586: 3293–3298. - PubMed
-
- Aebi M., Gassenhuber J., Domdey H., te Heesen S. (1996). Cloning and characterization of the ALG3 gene of Saccharomyces cerevisiae. Glycobiology 6: 439–444. - PubMed
-
- Bourett T.M., Sweigard J.A., Czymmek K.J., Carroll A., Howard R.J. (2002). Reef coral fluorescent proteins for visualizing fungal pathogens. Fungal Genet. Biol. 37: 211–220. - PubMed
-
- Bowman S.M., Free S.J. (2006). The structure and synthesis of the fungal cell wall. Bioessays 28: 799–808. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

