Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine
- PMID: 24643019
- PMCID: PMC3958361
- DOI: 10.1371/journal.pone.0089939
Antileishmanial activity of semisynthetic lupane triterpenoids betulin and betulinic acid derivatives: synergistic effects with miltefosine
Abstract
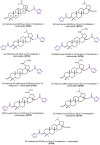
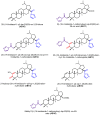
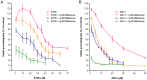
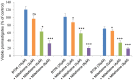
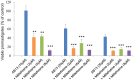
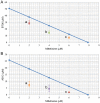
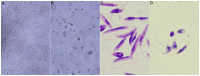
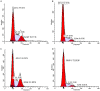
Leishmaniasis is a neglected tropical disease (NTDs), endemic in 88 countries, affecting more than 12 million people. The treatment consists in pentavalent antimony compounds, amphotericin B, pentamidine and miltefosine, among others. However, these current drugs are limited due to their toxicity, development of biological resistance, length of treatment and high cost. Thus, it is important to continue the search for new effective and less toxic treatments. The anti-Leishmania activity of sixteen semisynthetic lupane triterpenoids derivatives of betulin (BT01 to BT09) and betulinic acid (AB10 to AB16) were evaluated. Drug interactions between the active compounds and one current antileishmanial drug, miltefosine, were assessed using the fixed ratio isobologram method. In addition, effects on the cell cycle, apoptosis/necrosis events, morphology and DNA integrity were studied. The derivatives BT06 (3β-Hydroxy-(20R)-lupan-29-oxo-28-yl-1H-imidazole-1-carboxylate) and AB13 (28-(1H-imidazole-1-yl)-3,28-dioxo-lup-1,20(29)-dien-2-yl-1H-imidazole-1-carboxylate) were found to be the most active, with IC50 values of 50.8 µM and 25.8 µM, respectively. Interactions between these two compounds and miltefosine were classified as synergistic, with the most effective association being between AB13 and miltefosine, where decreases of IC50 values to 6 µM were observed, similar to the miltefosine activity alone. AB13 induced significant morphological changes, while both derivatives produced anti-proliferative activity through cell cycle arrest at the G0/G1 phase. Neither of these derivatives induced significant apoptosis/necrosis, as indicated by phosphatidylserine externalization and DNA fragmentation assays. In addition, neither of the derivatives induced death in macrophage cell lines. Thus, they do not present any potential risk of toxicity for the host cells. This study has identified the betulin derivative BT06 and the betulinic acid derivative AB13 as promising molecules in the development of new alternative therapies for leishmaniasis, including those involving combined-therapy with miltefosine.
Conflict of interest statement
Figures

References
-
- WHO (2010) Control of the leishmaniases. World Health Organization technical reportseries (949) xii–186, xii-xiii, 1-186. - PubMed
-
- Croft SL, Barrett MP, Urbina JA (2005) Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol 21 (11) 508–512. - PubMed
-
- Guerin PJ, Olliaro P, Sundar S, Boelaert M, Croft SL, et al. (2002) Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect Dis 2 (8) 494–501. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

