The Cosmc connection to the Tn antigen in cancer
- PMID: 24643043
- PMCID: PMC5808877
- DOI: 10.3233/CBM-130375
The Cosmc connection to the Tn antigen in cancer
Abstract
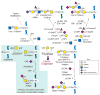
The Tn antigen is a tumor-associated carbohydrate antigen that is not normally expressed in peripheral tissues or blood cells. Expression of this antigen, which is found in a majority of human carcinomas of all types, arises from a blockage in the normal O-glycosylation pathway in which glycans are extended from the common precursor GalNAcα1-O-Ser/Thr (Tn antigen). This precursor is generated in the Golgi apparatus on newly synthesized glycoproteins by a family of polypeptide α-N-acetylgalactosaminyltransferases (ppGalNAcTs) and then extended to the common core 1 O-glycan Galβ1-3GalNAcα1-O-Ser/Thr (T antigen) by a single enzyme termed the T-synthase (core 1 β3-galactosyltransferase or C1GalT). Formation of the active form of the T-synthase requires a unique molecular chaperone termed Cosmc, encoded by Cosmc on the X-chromosome (Xq24 in humans, Xc3 in mice). Cosmc resides in the endoplasmic reticulum (ER) and prevents misfolding, aggregation, and proteasome-dependent degradation of newly synthesized T-synthase. Loss of expression of active T-synthase or Cosmc can lead to expression of the Tn antigen, along with its sialylated version Sialyl Tn antigen as observed in several cancers. Both genetic and epigenetic pathways, in addition to potential metabolic regulation, can result in abnormal expression of the Tn antigen. Engineered expression of the Tn antigen by disruption of either C1GalT (T-syn) or Cosmc in mice is associated with a tremendous range of pathologies and engineered expression of the Tn antigen in mouse embryos leads to embryonic death. Studies indicate that many membrane glycoproteins expressing the Tn antigen and/or truncated O-glycans may be dysfunctional, due to degradation and/or misfolding. Thus, expression of normal O-glycans is associated with health and homeostasis whereas truncation of O-glycans, e.g. the Tn and/or Sialyl Tn antigens is associated with cancer and other pathologies.
Keywords: Cosmc; T-synthase; Tn antigen; cancer.
Figures



Similar articles
-
Functional assays for the molecular chaperone cosmc.Methods Enzymol. 2010;479:107-22. doi: 10.1016/S0076-6879(10)79006-6. Methods Enzymol. 2010. PMID: 20816162
-
Epigenetic silencing of the chaperone Cosmc in human leukocytes expressing tn antigen.J Biol Chem. 2012 Nov 30;287(49):41523-33. doi: 10.1074/jbc.M112.371989. Epub 2012 Oct 3. J Biol Chem. 2012. PMID: 23035125 Free PMC article.
-
O-glycans on death receptors in cells modulate their sensitivity to TRAIL-induced apoptosis through affecting on their stability and oligomerization.FASEB J. 2020 Sep;34(9):11786-11801. doi: 10.1096/fj.201900053RR. Epub 2020 Jul 21. FASEB J. 2020. PMID: 32692906
-
The Tn antigen-structural simplicity and biological complexity.Angew Chem Int Ed Engl. 2011 Feb 18;50(8):1770-91. doi: 10.1002/anie.201002313. Epub 2011 Jan 21. Angew Chem Int Ed Engl. 2011. PMID: 21259410 Free PMC article. Review.
-
Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen.HLA. 2016 Dec;88(6):275-286. doi: 10.1111/tan.12900. Epub 2016 Sep 28. HLA. 2016. PMID: 27679419 Review.
Cited by
-
Biological roles of glycans.Glycobiology. 2017 Jan;27(1):3-49. doi: 10.1093/glycob/cww086. Epub 2016 Aug 24. Glycobiology. 2017. PMID: 27558841 Free PMC article. Review.
-
A roadmap for translational cancer glycoimmunology at single cell resolution.J Exp Clin Cancer Res. 2022 Apr 15;41(1):143. doi: 10.1186/s13046-022-02335-z. J Exp Clin Cancer Res. 2022. PMID: 35428302 Free PMC article. Review.
-
Demethylation of the Cosmc Promoter Alleviates the Progression of Breast Cancer Through Downregulation of the Tn and Sialyl-Tn Antigens.Cancer Manag Res. 2020 Feb 11;12:1017-1027. doi: 10.2147/CMAR.S214553. eCollection 2020. Cancer Manag Res. 2020. Retraction in: Cancer Manag Res. 2022 Nov 03;14:3119-3120. doi: 10.2147/CMAR.S395873. PMID: 32104083 Free PMC article. Retracted.
-
Large-Scale and Site-Specific Mapping of the Murine Brain O-Glycoproteome with IMPa.Anal Chem. 2023 Sep 12;95(36):13423-13430. doi: 10.1021/acs.analchem.3c00408. Epub 2023 Aug 25. Anal Chem. 2023. PMID: 37624755 Free PMC article.
-
Hallmarks of glycosylation in cancer.Oncotarget. 2016 Jun 7;7(23):35478-89. doi: 10.18632/oncotarget.8155. Oncotarget. 2016. PMID: 27007155 Free PMC article. Review.
References
-
- Eheman C, Henley SJ, Ballard-Barbash R, Jacobs EJ, Schymura MJ, Noone AM, Pan L, Anderson RN, Fulton JE, Kohler BA, Jemal A, Ward E, Plescia M, Ries LA, Edwards BK. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118(9):2338–66. - PMC - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: A looking glass for cancer? Nat Rev Cancer. 2012;12(5):323–34. - PubMed
-
- La Thangue NB, Kerr DJ. Predictive biomarkers: A paradigm shift towards personalized cancer medicine. Nat Rev Clin Oncol. 2011;8(10):587–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

