Caveolin-1 in lipid rafts interacts with dengue virus NS3 during polyprotein processing and replication in HMEC-1 cells
- PMID: 24643062
- PMCID: PMC3958351
- DOI: 10.1371/journal.pone.0090704
Caveolin-1 in lipid rafts interacts with dengue virus NS3 during polyprotein processing and replication in HMEC-1 cells
Abstract
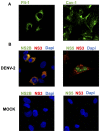
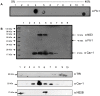
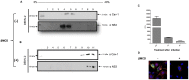
Lipid rafts are ordered microdomains within cellular membranes that are rich in cholesterol and sphingolipids. Caveolin (Cav-1) and flotillin (Flt-1) are markers of lipid rafts, which serve as an organizing center for biological phenomena and cellular signaling. Lipid rafts involvement in dengue virus (DENV) processing, replication, and assembly remains poorly characterized. Here, we investigated the role of lipid rafts after DENV endocytosis in human microvascular endothelial cells (HMEC-1). The non-structural viral proteins NS3 and NS2B, but not NS5, were associated with detergent-resistant membranes. In sucrose gradients, both NS3 and NS2B proteins appeared in Cav-1 and Flt-1 rich fractions. Additionally, double immunofluorescence staining of DENV-infected HMEC-1 cells showed that NS3 and NS2B, but not NS5, colocalized with Cav-1 and Flt-1. Furthermore, in HMEC-1cells transfected with NS3 protease, shown a strong overlap between NS3 and Cav-1, similar to that in DENV-infected cells. In contrast, double-stranded viral RNA (dsRNA) overlapped weakly with Cav-1 and Flt-1. Given these results, we investigated whether Cav-1 directly interacted with NS3. Cav-1 and NS3 co-immunoprecipitated, indicating that they resided within the same complex. Furthermore, when cellular cholesterol was depleted by methyl-beta cyclodextrin treatment after DENV entrance, lipid rafts were disrupted, NS3 protein level was reduced, besides Cav-1 and NS3 were displaced to fractions 9 and 10 in sucrose gradient analysis, and we observed a dramatically reduction of DENV particles release. These data demonstrate the essential role of caveolar cholesterol-rich lipid raft microdomains in DENV polyprotein processing and replication during the late stages of the DENV life cycle.
Conflict of interest statement
Figures




Similar articles
-
Monoclonal antibodies against dengue NS2B and NS3 proteins for the study of protein interactions in the flaviviral replication complex.J Virol Methods. 2012 Jan;179(1):97-103. doi: 10.1016/j.jviromet.2011.10.006. Epub 2011 Oct 20. J Virol Methods. 2012. PMID: 22040846
-
Dengue virus nonstructural 3 protein interacts directly with human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and reduces its glycolytic activity.Sci Rep. 2019 Feb 25;9(1):2651. doi: 10.1038/s41598-019-39157-7. Sci Rep. 2019. PMID: 30804377 Free PMC article.
-
A Combined Genetic-Proteomic Approach Identifies Residues within Dengue Virus NS4B Critical for Interaction with NS3 and Viral Replication.J Virol. 2015 Jul;89(14):7170-86. doi: 10.1128/JVI.00867-15. Epub 2015 Apr 29. J Virol. 2015. PMID: 25926641 Free PMC article.
-
The Transactions of NS3 and NS5 in Flaviviral RNA Replication.Adv Exp Med Biol. 2018;1062:147-163. doi: 10.1007/978-981-10-8727-1_11. Adv Exp Med Biol. 2018. PMID: 29845531 Review.
-
The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development.Antiviral Res. 2015 Jun;118:148-58. doi: 10.1016/j.antiviral.2015.03.014. Epub 2015 Apr 2. Antiviral Res. 2015. PMID: 25842996 Review.
Cited by
-
Recombinant Dengue virus protein NS2B alters membrane permeability in different membrane models.Virol J. 2016 Jan 4;13:1. doi: 10.1186/s12985-015-0456-4. Virol J. 2016. PMID: 26728778 Free PMC article.
-
System-oriented optimization of multi-target 2,6-diaminopurine derivatives: Easily accessible broad-spectrum antivirals active against flaviviruses, influenza virus and SARS-CoV-2.Eur J Med Chem. 2021 Nov 15;224:113683. doi: 10.1016/j.ejmech.2021.113683. Epub 2021 Jul 5. Eur J Med Chem. 2021. PMID: 34273661 Free PMC article.
-
Caveolae provide a specialized membrane environment for respiratory syncytial virus assembly.J Cell Sci. 2017 Mar 15;130(6):1037-1050. doi: 10.1242/jcs.198853. Epub 2017 Feb 2. J Cell Sci. 2017. PMID: 28154158 Free PMC article.
-
Inflammatory status and severity of disease in dengue patients are associated with lipoprotein alterations.PLoS One. 2019 Mar 22;14(3):e0214245. doi: 10.1371/journal.pone.0214245. eCollection 2019. PLoS One. 2019. PMID: 30901375 Free PMC article.
-
Regulation of Flavivirus RNA synthesis and replication.Curr Opin Virol. 2014 Dec;9:74-83. doi: 10.1016/j.coviro.2014.09.011. Epub 2014 Oct 17. Curr Opin Virol. 2014. PMID: 25462437 Free PMC article. Review.
References
-
- Lindenbach BD, Rice CM (2003) Molecular biology of flaviviruses. Adv Virus Res 59: 23–61. - PubMed
-
- Urcuqui-Inchima S, Patino C, Torres S, Haenni AL, Diaz FJ (2010) Recent developments in understanding dengue virus replication. Adv Virus Res 77: 1–39. - PubMed
-
- Tan BH, Fu J, Sugrue RJ, Yap EH, Chan YC, et al. (1996) Recombinant dengue type 1 virus NS5 protein expressed in Escherichia coli exhibits RNA-dependent RNA polymerase activity. Virology 216: 317–325. - PubMed
-
- Davidson AD (2009) Chapter 2. New insights into flavivirus nonstructural protein 5. Adv Virus Res 74: 41–101. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

