Microvesicular caspase-1 mediates lymphocyte apoptosis in sepsis
- PMID: 24643116
- PMCID: PMC3958341
- DOI: 10.1371/journal.pone.0090968
Microvesicular caspase-1 mediates lymphocyte apoptosis in sepsis
Abstract
Objective: Immune dysregulation during sepsis is poorly understood, however, lymphocyte apoptosis has been shown to correlate with poor outcomes in septic patients. The inflammasome, a molecular complex which includes caspase-1, is essential to the innate immune response to infection and also important in sepsis induced apoptosis. Our group has recently demonstrated that endotoxin-stimulated monocytes release microvesicles (MVs) containing caspase-1 that are capable of inducing apoptosis. We sought to determine if MVs containing caspase-1 are being released into the blood during human sepsis and induce apoptosis..
Design: Single-center cohort study.
Measurements: 50 critically ill patients were screened within 24 hours of admission to the intensive care unit and classified as either a septic or a critically ill control. Circulatory MVs were isolated and analyzed for the presence of caspase-1 and the ability to induce lymphocyte apoptosis. Patients remaining in the ICU for 48 hours had repeated measurement of caspase-1 activity on ICU day 3.
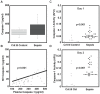
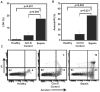
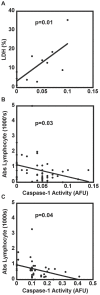
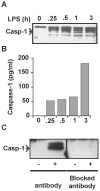
Main results: Septic patients had higher microvesicular caspase-1 activity 0.05 (0.04, 0.07) AFU versus 0.0 AFU (0, 0.02) (p<0.001) on day 1 and this persisted on day 3, 0.12 (0.1, 0.2) versus 0.02 (0, 0.1) (p<0.001). MVs isolated from septic patients on day 1 were able to induce apoptosis in healthy donor lymphocytes compared with critically ill control patients (17.8±9.2% versus 4.3±2.6% apoptotic cells, p<0.001) and depletion of MVs greatly diminished this apoptotic signal. Inhibition of caspase-1 or the disruption of MV integrity abolished the ability to induce apoptosis.
Conclusion: These findings suggest that microvesicular caspase-1 is important in the host response to sepsis, at least in part, via its ability to induce lymphocyte apoptosis. The ability of microvesicles to induce apoptosis requires active caspase-1 and intact microvesicles.
Conflict of interest statement
Figures

Similar articles
-
Microparticulate Caspase 1 Regulates Gasdermin D and Pulmonary Vascular Endothelial Cell Injury.Am J Respir Cell Mol Biol. 2018 Jul;59(1):56-64. doi: 10.1165/rcmb.2017-0393OC. Am J Respir Cell Mol Biol. 2018. PMID: 29365280 Free PMC article.
-
Endothelial microvesicles carrying Src-rich cargo impair adherens junction integrity and cytoskeleton homeostasis.Cardiovasc Res. 2020 Jul 1;116(8):1525-1538. doi: 10.1093/cvr/cvz238. Cardiovasc Res. 2020. PMID: 31504252 Free PMC article.
-
Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1.PLoS One. 2009 Sep 25;4(9):e7140. doi: 10.1371/journal.pone.0007140. PLoS One. 2009. PMID: 19779610 Free PMC article.
-
The pathogenesis of septic acute renal failure.Curr Opin Crit Care. 2003 Dec;9(6):496-502. doi: 10.1097/00075198-200312000-00006. Curr Opin Crit Care. 2003. PMID: 14639069 Review.
-
Assessing the immune status of critically ill trauma patients by flow cytometry.Nurs Res. 2014 Nov-Dec;63(6):426-34. doi: 10.1097/NNR.0000000000000061. Nurs Res. 2014. PMID: 25350542 Free PMC article. Review.
Cited by
-
Decoy Receptor 3 Improves Survival in Experimental Sepsis by Suppressing the Inflammatory Response and Lymphocyte Apoptosis.PLoS One. 2015 Jun 29;10(6):e0131680. doi: 10.1371/journal.pone.0131680. eCollection 2015. PLoS One. 2015. PMID: 26121476 Free PMC article.
-
Extracellular Vesicles: A Double-Edged Sword in Sepsis.Pharmaceuticals (Basel). 2021 Aug 23;14(8):829. doi: 10.3390/ph14080829. Pharmaceuticals (Basel). 2021. PMID: 34451925 Free PMC article. Review.
-
Francisella induced microparticulate caspase-1/gasdermin-D activation is regulated by NLRP3 independent of Pyrin.PLoS One. 2018 Dec 31;13(12):e0209931. doi: 10.1371/journal.pone.0209931. eCollection 2018. PLoS One. 2018. PMID: 30596757 Free PMC article.
-
Microparticulate Caspase 1 Regulates Gasdermin D and Pulmonary Vascular Endothelial Cell Injury.Am J Respir Cell Mol Biol. 2018 Jul;59(1):56-64. doi: 10.1165/rcmb.2017-0393OC. Am J Respir Cell Mol Biol. 2018. PMID: 29365280 Free PMC article.
-
Mononuclear Phagocyte-Derived Microparticulate Caspase-1 Induces Pulmonary Vascular Endothelial Cell Injury.PLoS One. 2015 Dec 28;10(12):e0145607. doi: 10.1371/journal.pone.0145607. eCollection 2015. PLoS One. 2015. PMID: 26710067 Free PMC article.
References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348: 1546–1554. - PubMed
-
- Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150. - PubMed
-
- Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, et al. (1999) Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med 27: 1230–1251. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

